Mycobacterium tuberculosis secretory proteins downregulate T cell activation by interfering with proximal and downstream T cell signalling events
- PMID: 26552486
- PMCID: PMC4640201
- DOI: 10.1186/s12865-015-0128-6
Mycobacterium tuberculosis secretory proteins downregulate T cell activation by interfering with proximal and downstream T cell signalling events
Abstract
Background: Mycobacterium tuberculosis (M. tuberculosis) modulates host immune response, mainly T cell responses for its own survival leading to disease or latent infection. The molecules and mechanisms utilized to accomplish immune subversion by M. tuberculosis are not fully understood. Understanding the molecular mechanism of T cell response to M. tuberculosis is important for development of efficacious vaccine against TB.
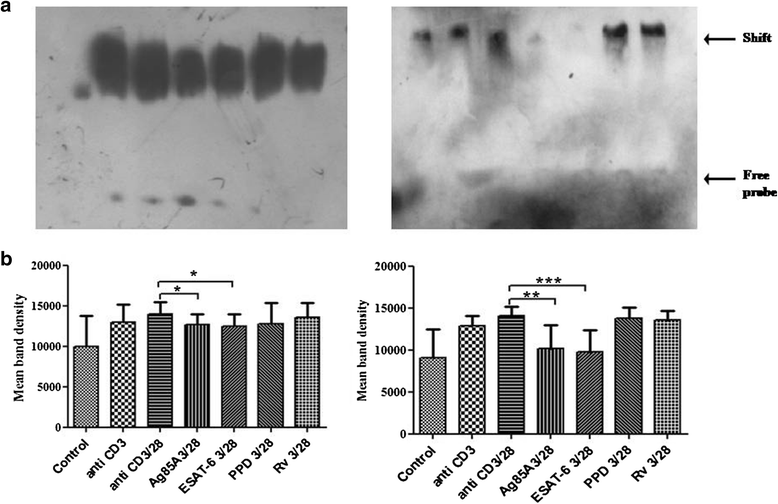
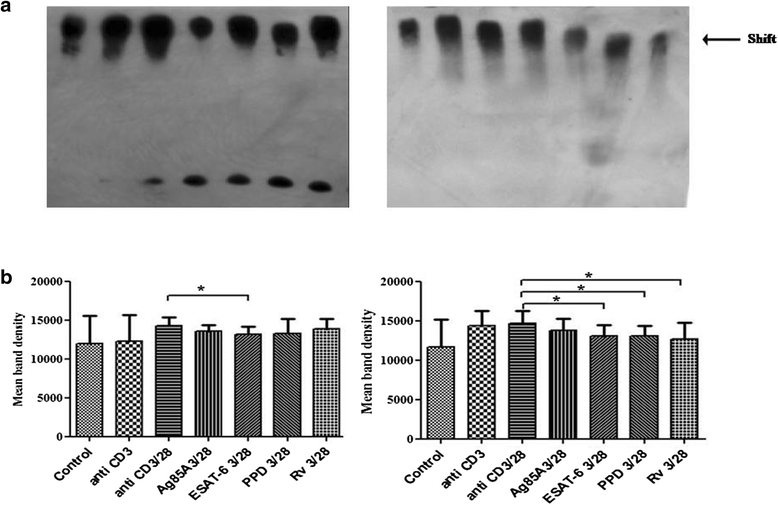
Methods: Here, we investigated effect of M. tuberculosis antigens Ag85A and ESAT-6 on T cell signalling events in CD3/CD28 induced Peripheral blood mononuclear cells (PBMCs) of PPD+ve healthy individuals and pulmonary TB patients. We studied CD3 induced intracellular calcium mobilization in PBMCs of healthy individuals and TB patients by spectrofluorimetry, CD3 and CD28 induced activation of mitogen activated protein kinases (MAPKs) in PBMCs of healthy individuals and TB patients by western blotting and binding of transcription factors NFAT and NFκB by Electrophorectic mobility shift assay (EMSA).
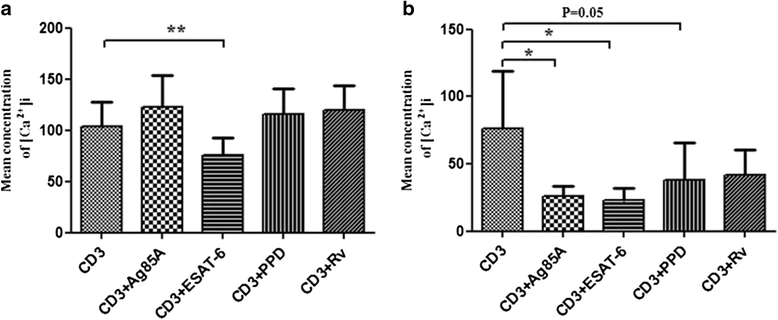
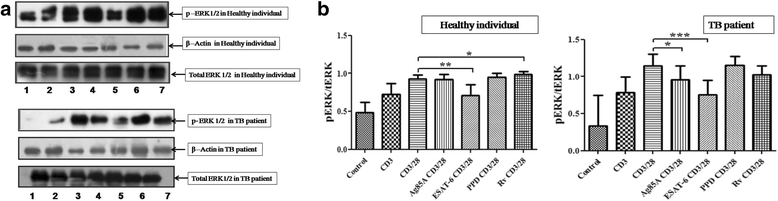
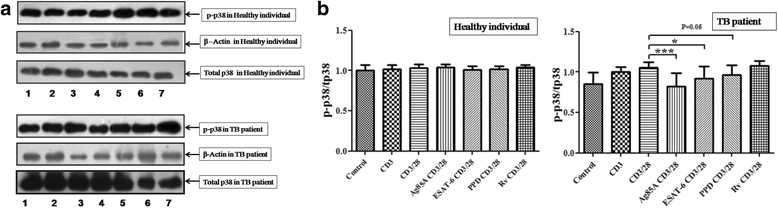
Results: We observed CD3 triggered modulations in free intracellular calcium concentrations in PPD+ve healthy individuals and pulmonary TB patients after the treatment of M. tuberculosis antigens. As regards the downstream signalling events, phosphorylation of MAPKs, Extracellular signal-regulated kinase 1 and 2 (ERK1/2) and p38 was curtailed by M. tuberculosis antigens in TB patients whereas, in PPD+ve healthy individuals only ERK1/2 phosphorylation was inhibited. Besides, the terminal signalling events like binding of transcription factors NFAT and NFκB was also altered by M. tuberculosis antigens. Altogether, our results suggest that M. tuberculosis antigens, specifically ESAT-6, interfere with TCR/CD28-induced upstream as well as downstream signalling events which might be responsible for defective IL-2 production which further contributed in T-cell unresponsiveness, implicated in the progression of disease.
Conclusion: To the best of our knowledge, this is the first study to investigate effect of Ag85A and ESAT-6 on TCR- and TCR/CD28- induced upstream and downstream signalling events of T-cell activation in TB patients. This study showed the effect of secretory antigens of M. tuberculosis in the modulation of T cell signalling pathways. This inflection is accomplished by altering the proximal and distal events of signalling cascade which could be involved in T-cell dysfunctioning during the progression of the disease.
Figures
Similar articles
-
Mycobacterial antigen(s) induce anergy by altering TCR- and TCR/CD28-induced signalling events: insights into T-cell unresponsiveness in leprosy.Mol Immunol. 2010 Feb;47(5):943-52. doi: 10.1016/j.molimm.2009.11.009. Epub 2009 Dec 16. Mol Immunol. 2010. PMID: 20018378
-
Investigating role of Mycobacterium tuberculosis secretory antigens in altering activation of T cell signaling events in Jurkat T cells.Int J Mycobacteriol. 2020 Oct-Dec;9(4):405-410. doi: 10.4103/ijmy.ijmy_172_20. Int J Mycobacteriol. 2020. PMID: 33323656
-
Phenolic-glycolipid-1 and lipoarabinomannan preferentially modulate TCR- and CD28-triggered proximal biochemical events, leading to T-cell unresponsiveness in mycobacterial diseases.Lipids Health Dis. 2012 Sep 17;11:119. doi: 10.1186/1476-511X-11-119. Lipids Health Dis. 2012. PMID: 22985026 Free PMC article.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
NF-κB family of transcription factors: biochemical players of CD28 co-stimulation.Immunol Lett. 2011 Mar 30;135(1-2):1-9. doi: 10.1016/j.imlet.2010.09.005. Epub 2010 Sep 21. Immunol Lett. 2011. PMID: 20863851 Review.
Cited by
-
The IL-17A rs2275913 single nucleotide polymorphism is associated with protection to tuberculosis but related to higher disease severity in Argentina.Sci Rep. 2017 Jan 18;7:40666. doi: 10.1038/srep40666. Sci Rep. 2017. PMID: 28098168 Free PMC article.
-
New Advances in the Development and Design of Mycobacterium tuberculosis Vaccines: Construction and Validation of Multi-Epitope Vaccines for Tuberculosis Prevention.Biology (Basel). 2025 Apr 13;14(4):417. doi: 10.3390/biology14040417. Biology (Basel). 2025. PMID: 40282282 Free PMC article. Review.
-
Changes in T-lymphocyte subsets and risk factors in human immunodeficiency virus-negative patients with active tuberculosis.Infection. 2020 Aug;48(4):585-595. doi: 10.1007/s15010-020-01451-2. Epub 2020 May 29. Infection. 2020. PMID: 32472529 Free PMC article.
-
Exploring modulations in T-cell receptor-mediated T-cell signaling events in systemic circulation and at local disease site of patients with tubercular pleural effusion: An attempt to understand tuberculosis pathogenesis at the local disease site.Front Med (Lausanne). 2022 Dec 1;9:983605. doi: 10.3389/fmed.2022.983605. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36530917 Free PMC article.
References
-
- World Health Organization . Global tuberculosis report. Geneva: WHO/HTM/TB; 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

