CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow
- PMID: 26552707
- PMCID: PMC4647260
- DOI: 10.1084/jem.20150057
CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow
Erratum in
-
Correction: CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow.J Exp Med. 2015 Dec 14;212(13):2323. doi: 10.1084/jem.2015005711172015c. Epub 2015 Dec 7. J Exp Med. 2015. PMID: 26642853 Free PMC article. No abstract available.
Abstract
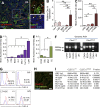
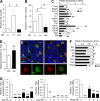
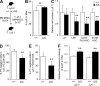
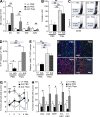
Hematopoietic stem cells (HSCs) depend on the bone marrow (BM) niche for their maintenance, proliferation, and differentiation. The BM niche is composed of nonhematopoietic and mature hematopoietic cells, including megakaryocytes (Mks). Thrombopoietin (Thpo) is a crucial cytokine produced by BM niche cells. However, the cellular source of Thpo, upon which HSCs primarily depend, is unclear. Moreover, no specific molecular pathway for the regulation of Thpo production in the BM has been identified. Here, we demonstrate that the membrane protein C-type lectin-like receptor-2 (CLEC-2) mediates the production of Thpo and other factors in Mks. Mice conditionally deleted for CLEC-2 in Mks (Clec2(MkΔ/Δ)) produced lower levels of Thpo in Mks. CLEC-2-deficient Mks showed down-regulation of CLEC-2-related signaling molecules Syk, Lcp2, and Plcg2. Knockdown of these molecules in cultured Mks decreased expression of Thpo. Clec2(MkΔ/Δ) mice exhibited reduced BM HSC quiescence and repopulation potential, along with extramedullary hematopoiesis. The low level of Thpo production may account for the decline in HSC potential in Clec2(MkΔ/Δ) mice, as administration of recombinant Thpo to Clec2(MkΔ/Δ) mice restored stem cell potential. Our study identifies CLEC-2 signaling as a novel molecular mechanism mediating the production of Thpo and other factors for the maintenance of HSCs.
© 2015 Nakamura-Ishizu et al.
Figures




Similar articles
-
Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin.Biochem Biophys Res Commun. 2014 Nov 14;454(2):353-7. doi: 10.1016/j.bbrc.2014.10.095. Epub 2014 Oct 24. Biochem Biophys Res Commun. 2014. PMID: 25451253
-
Niche regulation of hematopoietic stem cells in the endosteum.Ann N Y Acad Sci. 2009 Sep;1176:36-46. doi: 10.1111/j.1749-6632.2009.04561.x. Ann N Y Acad Sci. 2009. PMID: 19796231
-
Thrombopoietin from hepatocytes promotes hematopoietic stem cell regeneration after myeloablation.Elife. 2021 Aug 31;10:e69894. doi: 10.7554/eLife.69894. Elife. 2021. PMID: 34463253 Free PMC article.
-
The bone marrow niche from the inside out: how megakaryocytes are shaped by and shape hematopoiesis.Blood. 2022 Jan 27;139(4):483-491. doi: 10.1182/blood.2021012827. Blood. 2022. PMID: 34587234 Free PMC article. Review.
-
[Thrombopoietin regulates mitochondria homeostasis for hematopoietic stem cell maintenance].Rinsho Ketsueki. 2021;62(5):521-527. doi: 10.11406/rinketsu.62.521. Rinsho Ketsueki. 2021. PMID: 34248130 Review. Japanese.
Cited by
-
Cobalt hematoporphyrin inhibits CLEC-2-podoplanin interaction, tumor metastasis, and arterial/venous thrombosis in mice.Blood Adv. 2018 Sep 11;2(17):2214-2225. doi: 10.1182/bloodadvances.2018016261. Blood Adv. 2018. PMID: 30190281 Free PMC article.
-
Circular RNA ATAD1 is upregulated in acute myeloid leukemia and promotes cancer cell proliferation by downregulating miR-34b via promoter methylation.Oncol Lett. 2021 Nov;22(5):799. doi: 10.3892/ol.2021.13060. Epub 2021 Sep 20. Oncol Lett. 2021. PMID: 34630706 Free PMC article.
-
Niches for Hematopoietic Stem Cells and Their Progeny.Immunity. 2018 Apr 17;48(4):632-648. doi: 10.1016/j.immuni.2018.03.024. Immunity. 2018. PMID: 29669248 Free PMC article. Review.
-
Obesity-induced inflammation: The impact of the hematopoietic stem cell niche.JCI Insight. 2021 Feb 8;6(3):e145295. doi: 10.1172/jci.insight.145295. JCI Insight. 2021. PMID: 33554957 Free PMC article. Review.
-
Inflammation as a driver of hematological malignancies.Front Oncol. 2024 Mar 20;14:1347402. doi: 10.3389/fonc.2024.1347402. eCollection 2024. Front Oncol. 2024. PMID: 38571491 Free PMC article. Review.
References
-
- Acton S.E., Astarita J.L., Malhotra D., Lukacs-Kornek V., Franz B., Hess P.R., Jakus Z., Kuligowski M., Fletcher A.L., Elpek K.G., et al. . 2012. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity. 37:276–289. 10.1016/j.immuni.2012.05.022 - DOI - PMC - PubMed
-
- Acton S.E., Farrugia A.J., Astarita J.L., Mourão-Sá D., Jenkins R.P., Nye E., Hooper S., van Blijswijk J., Rogers N.C., Snelgrove K.J., et al. . 2014. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature. 514:498–502. 10.1038/nature13814 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

