Near-infrared (NIR) up-conversion optogenetics
- PMID: 26552717
- PMCID: PMC4639720
- DOI: 10.1038/srep16533
Near-infrared (NIR) up-conversion optogenetics
Abstract
Non-invasive remote control technologies designed to manipulate neural functions have been long-awaited for the comprehensive and quantitative understanding of neuronal network in the brain as well as for the therapy of neurological disorders. Recently, it has become possible for the neuronal activity to be optically manipulated using biological photo-reactive molecules such as channelrhodopsin (ChR)-2. However, ChR2 and its relatives are mostly reactive to visible light, which does not effectively penetrate through biological tissues. In contrast, near-infrared (NIR) light (650-1450 nm) penetrates deep into the tissues because biological systems are almost transparent to light within this so-called 'imaging window'. Here we used lanthanide nanoparticles (LNPs), composed of rare-earth elements, as luminous bodies to activate ChRs since they absorb low-energy NIR light to emit high-energy visible light (up-conversion). Here, we created a new type of optogenetic system which consists of the donor LNPs and the acceptor ChRs. The NIR laser irradiation emitted visible light from LNPs, then induced the photo-reactive responses in the near-by cells that expressed ChRs. However, there remains room for large improvements in the energy efficiency of the LNP-ChR system.
Figures
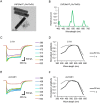

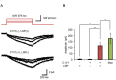
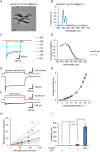
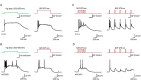
References
-
- Boyden E.S., Zhang F., Bamberg E., Nagel G. & Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005). - PubMed
-
- Ishizuka T., Kakuda M., Araki R. & Yawo H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci. Res. 54, 85–94 (2006). - PubMed
-
- Zhang F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

