In vivo quantitative imaging of photoassimilate transport dynamics and allocation in large plants using a commercial positron emission tomography (PET) scanner
- PMID: 26552889
- PMCID: PMC4640171
- DOI: 10.1186/s12870-015-0658-3
In vivo quantitative imaging of photoassimilate transport dynamics and allocation in large plants using a commercial positron emission tomography (PET) scanner
Abstract
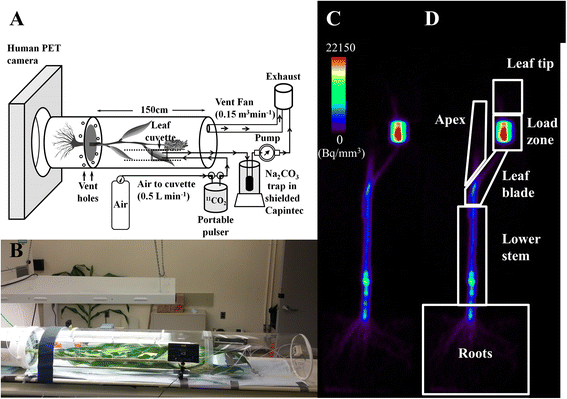
Background: Although important aspects of whole-plant carbon allocation in crop plants (e.g., to grain) occur late in development when the plants are large, techniques to study carbon transport and allocation processes have not been adapted for large plants. Positron emission tomography (PET), developed for dynamic imaging in medicine, has been applied in plant studies to measure the transport and allocation patterns of carbohydrates, nutrients, and phytohormones labeled with positron-emitting radioisotopes. However, the cost of PET and its limitation to smaller plants has restricted its use in plant biology. Here we describe the adaptation and optimization of a commercial clinical PET scanner to measure transport dynamics and allocation patterns of (11)C-photoassimilates in large crops.
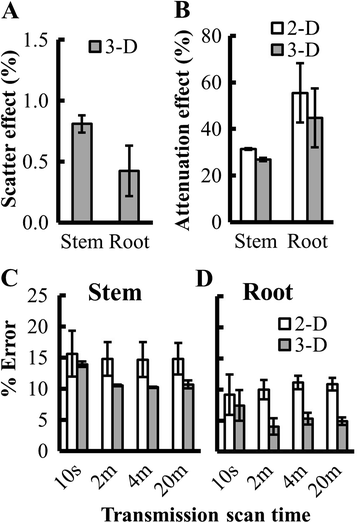
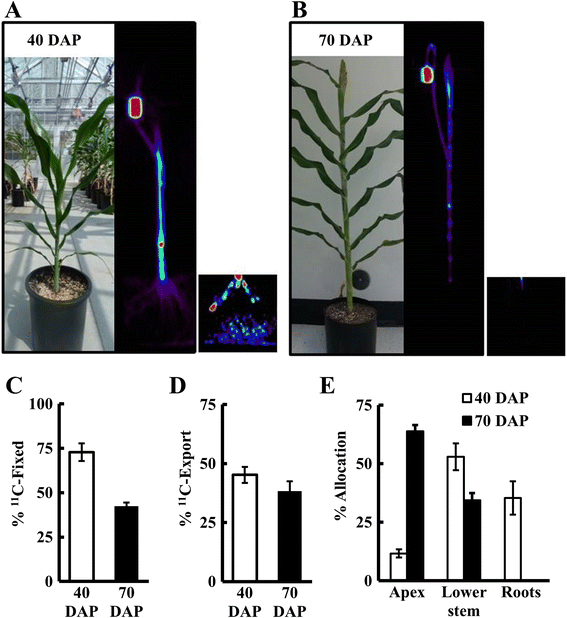
Results: Based on measurements of a phantom, we optimized instrument settings, including use of 3-D mode and attenuation correction to maximize the accuracy of measurements. To demonstrate the utility of PET, we measured (11)C-photoassimilate transport and allocation in Sorghum bicolor, an important staple crop, at vegetative and reproductive stages (40 and 70 days after planting; DAP). The (11)C-photoassimilate transport speed did not change over the two developmental stages. However, within a stem, transport speeds were reduced across nodes, likely due to higher (11)C-photoassimilate unloading in the nodes. Photosynthesis in leaves and the amount of (11)C that was exported to the rest of the plant decreased as plants matured. In young plants, exported (11)C was allocated mostly (88 %) to the roots and stem, but in flowering plants (70 DAP) the majority of the exported (11)C (64 %) was allocated to the apex.
Conclusions: Our results show that commercial PET scanners can be used reliably to measure whole-plant C-allocation in large plants nondestructively including, importantly, allocation to roots in soil. This capability revealed extreme changes in carbon allocation in sorghum plants, as they advanced to maturity. Further, our results suggest that nodes may be important control points for photoassimilate distribution in crops of the family Poaceae. Quantifying real-time carbon allocation and photoassimilate transport dynamics, as demonstrated here, will be important for functional genomic studies to unravel the mechanisms controlling phloem transport in large crop plants, which will provide crucial insights for improving yields.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

