Population Pharmacokinetics and Pharmacodynamics of Extended-Infusion Piperacillin and Tazobactam in Critically Ill Children
- PMID: 26552978
- PMCID: PMC4704193
- DOI: 10.1128/AAC.02089-15
Population Pharmacokinetics and Pharmacodynamics of Extended-Infusion Piperacillin and Tazobactam in Critically Ill Children
Abstract
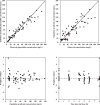
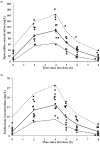
The study objective was to evaluate the population pharmacokinetics and pharmacodynamics of extended-infusion piperacillin-tazobactam in children hospitalized in an intensive care unit. Seventy-two serum samples were collected at steady state from 12 patients who received piperacillin-tazobactam at 100/12.5 mg/kg of body weight every 8 h infused over 4 h. Population pharmacokinetic analyses were performed using NONMEM, and Monte Carlo simulations were performed to estimate the piperacillin pharmacokinetic profiles for dosing regimens of 80 to 100 mg/kg of the piperacillin component given every 6 to 8 h and infused over 0.5, 3, or 4 h. The probability of target attainment (PTA) for a cumulative percentage of the dosing interval that the drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (TMIC) of ≥50% was calculated at MICs ranging from 0.25 to 64 mg/liter. The mean ± standard deviation (SD) age, weight, and estimated glomerular filtration rate were 5 ± 3 years, 17 ± 6.2 kg, and 118 ± 41 ml/min/1.73 m(2), respectively. A one-compartment model with zero-order input and first-order elimination best fit the pharmacokinetic data for both drugs. Weight was significantly associated with piperacillin clearance, and weight and sex were significantly associated with tazobactam clearance. Pharmacokinetic parameters (mean ± SD) for piperacillin and tazobactam were as follows: clearance, 0.22 ± 0.07 and 0.19 ± 0.07 liter/h/kg, respectively; volume of distribution, 0.43 ± 0.16 and 0.37 ± 0.14 liter/kg, respectively. All extended-infusion regimens achieved PTAs of >90% at MICs of ≤16 mg/liter. Only the 3-h infusion regimens given every 6 h achieved PTAs of >90% at an MIC of 32 mg/liter. For susceptible bacterial pathogens, piperacillin-tazobactam doses of ≥80/10 mg/kg given every 8 h and infused over 4 h achieve adequate pharmacodynamic exposures in critically ill children.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

