Structure and function of the septum nasi and the underlying tension chord in crocodylians
- PMID: 26552989
- PMCID: PMC4694159
- DOI: 10.1111/joa.12404
Structure and function of the septum nasi and the underlying tension chord in crocodylians
Abstract
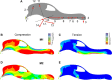
A long rostrum has distinct advantages for prey capture in an aquatic or semi-aquatic environment but at the same time poses severe problems concerning stability during biting. We here investigate the role of the septum nasi of brevirostrine crocodilians for load-absorption during mastication. Histologically, both the septum nasi and the septum interorbitale consist of hyaline cartilage and therefore mainly resist compression. However, we identified a strand of tissue extending longitudinally below the septum nasi that is characterized by a high content of collagenous and elastic fibers and could therefore resist tensile stresses. This strand of tissue is connected with the m. pterygoideus anterior. Two-dimensional finite element modeling shows that minimization of bending in the crocodilian skull can only be achieved if tensile stresses are counteracted by a strand of tissue. We propose that the newly identified strand of tissue acts as an active tension chord necessary for stabilizing the long rostrum of crocodilians during biting by transforming the high bending stress of the rostrum into moderate compressive stress.
Keywords: finite element analysis; histology; septum nasi; tension chord.
© 2015 Anatomical Society.
Figures







Similar articles
-
Compressive and tensile mechanical properties of the porcine nasal septum.J Biomech. 2014 Jan 3;47(1):154-61. doi: 10.1016/j.jbiomech.2013.09.026. Epub 2013 Oct 8. J Biomech. 2014. PMID: 24268797 Free PMC article.
-
The biomechanical consequences of longirostry in crocodilians and odontocetes.J Biomech. 2017 May 3;56:61-70. doi: 10.1016/j.jbiomech.2017.03.003. Epub 2017 Mar 21. J Biomech. 2017. PMID: 28363382
-
Biomechanics of the rostrum in crocodilians: a comparative analysis using finite-element modeling.Anat Rec A Discov Mol Cell Evol Biol. 2006 Aug;288(8):827-49. doi: 10.1002/ar.a.20360. Anat Rec A Discov Mol Cell Evol Biol. 2006. PMID: 16835925
-
Tooth-PDL-bone complex: response to compressive loads encountered during mastication - a review.Arch Oral Biol. 2012 Dec;57(12):1575-84. doi: 10.1016/j.archoralbio.2012.07.006. Epub 2012 Aug 9. Arch Oral Biol. 2012. PMID: 22877793 Review.
-
The role of computational models in the search for the mechanical behavior and damage mechanisms of articular cartilage.Med Eng Phys. 2005 Dec;27(10):810-26. doi: 10.1016/j.medengphy.2005.03.004. Med Eng Phys. 2005. PMID: 16287601 Review.
Cited by
-
The biomechanical role of the chondrocranium and sutures in a lizard cranium.J R Soc Interface. 2017 Dec;14(137):20170637. doi: 10.1098/rsif.2017.0637. J R Soc Interface. 2017. PMID: 29263126 Free PMC article.
-
Trifold origin of the reptilian ear ossicle and its relation to the evolutionary modification of the temporal skull region.J Anat. 2025 Mar;246(3):402-414. doi: 10.1111/joa.14105. Epub 2024 Sep 19. J Anat. 2025. PMID: 39297283 Free PMC article.
-
Synchrotron "virtual archaeozoology" reveals how Ancient Egyptians prepared a decaying crocodile cadaver for mummification.PLoS One. 2020 Feb 21;15(2):e0229140. doi: 10.1371/journal.pone.0229140. eCollection 2020. PLoS One. 2020. PMID: 32084197 Free PMC article.
-
Determination of muscle strength and function in plesiosaur limbs: finite element structural analyses of Cryptoclidus eurymerus humerus and femur.PeerJ. 2022 Jun 3;10:e13342. doi: 10.7717/peerj.13342. eCollection 2022. PeerJ. 2022. PMID: 35677394 Free PMC article.
-
Septal deviation in the nose of the longest faced crocodylian: A description of nasal anatomy and airflow in the Indian gharial (Gavialis gangeticus) with comments on acoustics.Anat Rec (Hoboken). 2022 Oct;305(10):2883-2903. doi: 10.1002/ar.24831. Epub 2021 Nov 23. Anat Rec (Hoboken). 2022. PMID: 34813139 Free PMC article.
References
-
- Babula WJ, Smiley GR, Dixon AD (1970) The role of the cartilaginous nasal septum in midfacial growth. Am J Orthod 58, 250–263. - PubMed
-
- Bona P, Desojo JB (2011) Osteology and cranial musculature of Caiman latirostris (Crocodylia: Alligatoridae). J Morphol 272, 780–795. - PubMed
-
- Brochu CA (2003) Phylogenetic approaches toward crocodilian history. Annu Rev Earth Planet Sci 31, 357–397.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

