Transplantation of rat embryonic stem cell-derived retinal progenitor cells preserves the retinal structure and function in rat retinal degeneration
- PMID: 26553210
- PMCID: PMC4640237
- DOI: 10.1186/s13287-015-0207-x
Transplantation of rat embryonic stem cell-derived retinal progenitor cells preserves the retinal structure and function in rat retinal degeneration
Abstract
Introduction: Degenerative retinal diseases like age-related macular degeneration (AMD) are the leading cause of blindness. Cell transplantation showed promising therapeutic effect for such diseases, and embryonic stem cell (ESC) is one of the sources of such donor cells. Here, we aimed to generate retinal progenitor cells (RPCs) from rat ESCs (rESCs) and to test their therapeutic effects in rat model.
Methods: The rESCs (DA8-16) were cultured in N2B27 medium with 2i, and differentiated to two types of RPCs following the SFEBq method with modifications. For rESC-RPC1, the cells were switched to adherent culture at D10, while for rESC-RPC2, the suspension culture was maintained to D14. Both RPCs were harvested at D16. Primary RPCs were obtained from P1 SD rats, and some of them were labeled with EGFP by infection with lentivirus. To generate Rax::EGFP knock-in rESC lines, TALENs were engineered to facilitate homologous recombination in rESCs, which were cotransfected with the targeting vector and TALEN vectors. The differentiated cells were analyzed with live image, immunofluorescence staining, flow cytometric analysis, gene expression microarray, etc. RCS rats were used to mimic the degeneration of retina and test the therapeutic effects of subretinally transplanted donor cells. The structure and function of retina were examined.
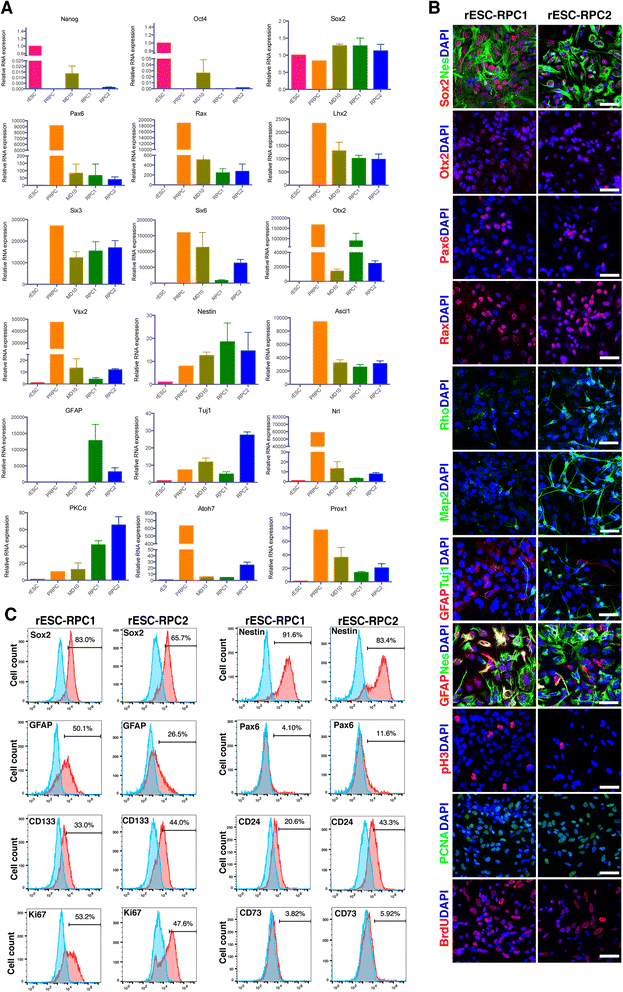
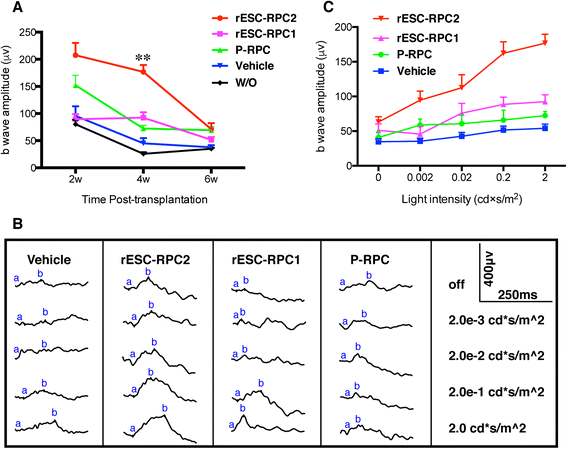
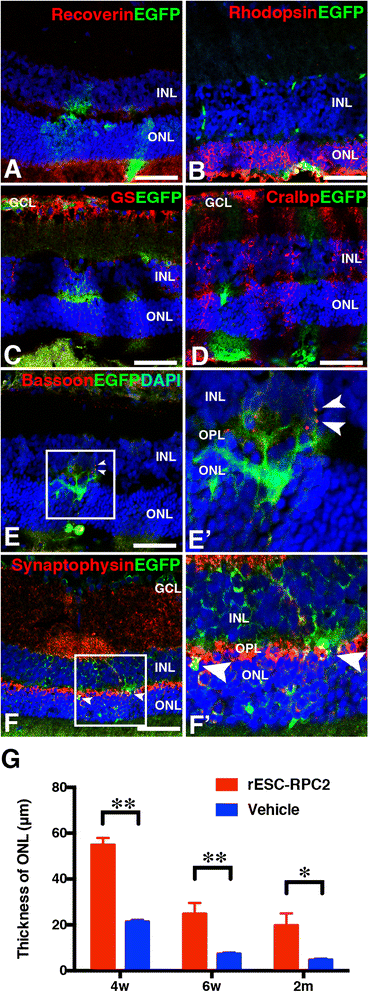
Results: We established two protocols through which two types of rESC-derived RPCs were obtained and both contained committed retina lineage cells and some neural progenitor cells (NPCs). These rESC-derived RPCs survived in the host retinas of RCS rats and protected the retinal structure and function in early stage following the transplantation. However, the glia enriched rESC-RPC1 obtained through early and longer adherent culture only increased the b-wave amplitude at 4 weeks, while the longer suspension culture gave rise to evidently neuronal differentiation in rESC-RPC2 which significantly improved the visual function of RCS rats.
Conclusions: We have successfully differentiated rESCs to glia enriched RPCs and retinal neuron enriched RPCs in vitro. The retinal neuron enriched rESC-RPC2 protected the structure and function of retina in rats with genetic retinal degeneration and could be a candidate cell source for treating some degenerative retinal diseases in human trials.
Figures



Similar articles
-
Transplantation of rat embryonic stem cell-derived retinal cells restores visual function in the Royal College of Surgeons rats.Doc Ophthalmol. 2018 Oct;137(2):71-78. doi: 10.1007/s10633-018-9648-8. Epub 2018 Aug 3. Doc Ophthalmol. 2018. PMID: 30074097
-
Isolation of retinal progenitor and stem cells from the porcine eye.Mol Vis. 2007 Jun 29;13:1045-57. Mol Vis. 2007. PMID: 17653049 Free PMC article.
-
Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats.Exp Eye Res. 2005 Apr;80(4):515-25. doi: 10.1016/j.exer.2004.11.001. Exp Eye Res. 2005. PMID: 15781279
-
Stem/progenitor cell-based transplantation for retinal degeneration: a review of clinical trials.Cell Death Dis. 2020 Sep 23;11(9):793. doi: 10.1038/s41419-020-02955-3. Cell Death Dis. 2020. PMID: 32968042 Free PMC article. Review.
-
Towards Stem/Progenitor Cell-Based Therapies for Retinal Degeneration.Stem Cell Rev Rep. 2024 Aug;20(6):1459-1479. doi: 10.1007/s12015-024-10740-4. Epub 2024 May 29. Stem Cell Rev Rep. 2024. PMID: 38809490 Review.
Cited by
-
Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia.J Extracell Vesicles. 2020 Apr 21;9(1):1748931. doi: 10.1080/20013078.2020.1748931. eCollection 2020. J Extracell Vesicles. 2020. PMID: 32373289 Free PMC article.
-
Subretinal Human Umbilical Tissue-Derived Cell Transplantation Preserves Retinal Synaptic Connectivity and Attenuates Müller Glial Reactivity.J Neurosci. 2018 Mar 21;38(12):2923-2943. doi: 10.1523/JNEUROSCI.1532-17.2018. Epub 2018 Feb 5. J Neurosci. 2018. PMID: 29431645 Free PMC article.
-
Ocular Stem Cell Research from Basic Science to Clinical Application: A Report from Zhongshan Ophthalmic Center Ocular Stem Cell Symposium.Int J Mol Sci. 2016 Mar 22;17(3):415. doi: 10.3390/ijms17030415. Int J Mol Sci. 2016. PMID: 27102165 Free PMC article.
-
Experimental Study of the Biological Properties of Human Embryonic Stem Cell-Derived Retinal Progenitor Cells.Sci Rep. 2017 Feb 13;7:42363. doi: 10.1038/srep42363. Sci Rep. 2017. PMID: 28205557 Free PMC article.
-
Combined Transplantation With Human Mesenchymal Stem Cells Improves Retinal Rescue Effect of Human Fetal RPE Cells in Retinal Degeneration Mouse Model.Invest Ophthalmol Vis Sci. 2020 Jul 1;61(8):9. doi: 10.1167/iovs.61.8.9. Invest Ophthalmol Vis Sci. 2020. PMID: 32639552 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

