mActive: A Randomized Clinical Trial of an Automated mHealth Intervention for Physical Activity Promotion
- PMID: 26553211
- PMCID: PMC4845232
- DOI: 10.1161/JAHA.115.002239
mActive: A Randomized Clinical Trial of an Automated mHealth Intervention for Physical Activity Promotion
Abstract
Background: We hypothesized that a fully automated mobile health (mHealth) intervention with tracking and texting components would increase physical activity.
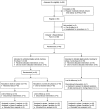
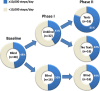
Methods and results: mActive enrolled smartphone users aged 18 to 69 years at an ambulatory cardiology center in Baltimore, Maryland. We used sequential randomization to evaluate the intervention's 2 core components. After establishing baseline activity during a blinded run-in (week 1), in phase I (weeks 2 to 3), we randomized 2:1 to unblinded versus blinded tracking. Unblinding allowed continuous access to activity data through a smartphone interface. In phase II (weeks 4 to 5), we randomized unblinded participants 1:1 to smart texts versus no texts. Smart texts provided smartphone-delivered coaching 3 times/day aimed at individual encouragement and fostering feedback loops by a fully automated, physician-written, theory-based algorithm using real-time activity data and 16 personal factors with a 10 000 steps/day goal. Forty-eight outpatients (46% women, 21% nonwhite) enrolled with a mean±SD age of 58±8 years, body mass index of 31±6 kg/m(2), and baseline activity of 9670±4350 steps/day. Daily activity data capture was 97.4%. The phase I change in activity was nonsignificantly higher in unblinded participants versus blinded controls by 1024 daily steps (95% confidence interval [CI], -580 to 2628; P=0.21). In phase II, participants receiving texts increased their daily steps over those not receiving texts by 2534 (95% CI, 1318 to 3750; P<0.001) and over blinded controls by 3376 (95% CI, 1951 to 4801; P<0.001).
Conclusions: An automated tracking-texting intervention increased physical activity with, but not without, the texting component. These results support new mHealth tracking technologies as facilitators in need of behavior change drivers.
Clinical trial registration: URL: http://ClinicalTrials.gov/. Unique identifier: NCT01917812.
Keywords: accelerometer; activity tracker; automation; cardiovascular disease; digital health; eHealth; health technology; mHealth; mobile phone; pedometer; physical activity; prevention; smartphone; text messages; texting; wearable device; wearable sensor.
© 2015 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures
References
-
- Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;2014(129):S76–S99. - PubMed
-
- Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. - PubMed
-
- Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313:625–626. - PubMed
-
- Steinhubl SR, Muse ED, Topol EJ. Can mobile health technologies transform health care? JAMA. 2013;310:2395–2396. - PubMed

