Regulation of Primary Metabolism in Response to Low Oxygen Availability as Revealed by Carbon and Nitrogen Isotope Redistribution
- PMID: 26553649
- PMCID: PMC4704563
- DOI: 10.1104/pp.15.00266
Regulation of Primary Metabolism in Response to Low Oxygen Availability as Revealed by Carbon and Nitrogen Isotope Redistribution
Abstract
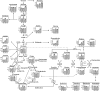
Based on enzyme activity assays and metabolic responses to waterlogging of the legume Lotus japonicus, it was previously suggested that, during hypoxia, the tricarboxylic acid cycle switches to a noncyclic operation mode. Hypotheses were postulated to explain the alternative metabolic pathways involved, but as yet, a direct analysis of the relative redistribution of label through the corresponding pathways was not made. Here, we describe the use of stable isotope-labeling experiments for studying metabolism under hypoxia using wild-type roots of the crop legume soybean (Glycine max). [(13)C]Pyruvate labeling was performed to compare metabolism through the tricarboxylic acid cycle, fermentation, alanine metabolism, and the γ-aminobutyric acid shunt, while [(13)C]glutamate and [(15)N]ammonium labeling were performed to address the metabolism via glutamate to succinate. Following these labelings, the time course for the redistribution of the (13)C/(15)N label throughout the metabolic network was evaluated with gas chromatography-time of flight-mass spectrometry. Our combined labeling data suggest the inhibition of the tricarboxylic acid cycle enzyme succinate dehydrogenase, also known as complex II of the mitochondrial electron transport chain, providing support for the bifurcation of the cycle and the down-regulation of the rate of respiration measured during hypoxic stress. Moreover, up-regulation of the γ-aminobutyric acid shunt and alanine metabolism explained the accumulation of succinate and alanine during hypoxia.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Araújo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR (2012) Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ 35: 1–21 - PubMed
-
- Araújo WL, Nunes-Nesi A, Osorio S, Usadel B, Fuentes D, Nagy R, Balbo I, Lehmann M, Studart-Witkowski C, Tohge T, et al. (2011) Antisense inhibition of the iron-sulphur subunit of succinate dehydrogenase enhances photosynthesis and growth in tomato via an organic acid-mediated effect on stomatal aperture. Plant Cell 23: 600–627 - PMC - PubMed
-
- Armstrong W, Beckett PM (2011) Experimental and modelling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase. New Phytol 190: 431–441 - PubMed
-
- Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LA, van Dongen JT (2012) Making sense of low oxygen sensing. Trends Plant Sci 17: 129–138 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

