Introduction and Rollout of a New Group A Meningococcal Conjugate Vaccine (PsA-TT) in African Meningitis Belt Countries, 2010-2014
- PMID: 26553672
- PMCID: PMC4639493
- DOI: 10.1093/cid/civ551
Introduction and Rollout of a New Group A Meningococcal Conjugate Vaccine (PsA-TT) in African Meningitis Belt Countries, 2010-2014
Abstract
Background: A group A meningococcal conjugate vaccine (PsA-TT) was developed specifically for the African "meningitis belt" and was prequalified by the World Health Organization (WHO) in June 2010. The vaccine was first used widely in Burkina Faso, Mali, and Niger in December 2010 with great success. The remaining 23 meningitis belt countries wished to use this new vaccine.
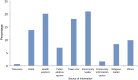
Methods: With the help of African countries, WHO developed a prioritization scheme and used or adapted existing immunization guidelines to mount PsA-TT vaccination campaigns. Vaccine requirements were harmonized with the Serum Institute of India, Ltd.
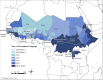
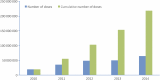
Results: Burkina Faso was the first country to fully immunize its 1- to 29-year-old population in December 2010. Over the next 4 years, vaccine coverage was extended to 217 million Africans living in 15 meningitis belt countries.
Conclusions: The new group A meningococcal conjugate vaccine was well received, with country coverage rates ranging from 85% to 95%. The rollout proceeded smoothly because countries at highest risk were immunized first while attention was paid to geographic contiguity to maximize herd protection. Community participation was exemplary.
Keywords: Africa; PsA-TT; meningitis belt; meningococcal group A; rollout plan.
© 2015 World Health Organization; licensee Oxford Journals.
Figures
References
-
- LaForce FM, Kondé K, Viviani S, Préziosi MP. The Meningitis Vaccine Project. Vaccine 2007; 25(suppl 1):A97–100. - PubMed
-
- Sow SO, Okoko BJ, Diallo A et al. . Immunogenicity and safety of a meningococcal A conjugate vaccine in Africa. N Engl J Med 2011; 364:2293–304. - PubMed
-
- Kristiansen PA, Diomandé F, Ba AK et al. . Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd protection. Clin Infect Dis 2012; 56:354–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

