Antibody Persistence at 1 and 4 Years Following a Single Dose of MenAfriVac or Quadrivalent Polysaccharide Vaccine in Healthy Subjects Aged 2-29 Years
- PMID: 26553684
- PMCID: PMC4639491
- DOI: 10.1093/cid/civ518
Antibody Persistence at 1 and 4 Years Following a Single Dose of MenAfriVac or Quadrivalent Polysaccharide Vaccine in Healthy Subjects Aged 2-29 Years
Abstract
Background: Mass vaccination campaigns of the population aged 1-29 years with 1 dose of group A meningococcal (MenA) conjugate vaccine (PsA-TT, MenAfriVac) in African meningitis belt countries has resulted in the near-disappearance of MenA. The vaccine was tested in clinical trials in Africa and in India and found to be safe and highly immunogenic compared with the group A component of the licensed quadrivalent polysaccharide vaccine (PsACWY). Antibody persistence in Africa and in India was investigated.
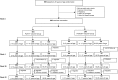
Methods: A total of 900 subjects aged 2-29 years were followed up for 4 years in Senegal, Mali, and The Gambia (study A). A total of 340 subjects aged 2-10 years were followed up for 1 year in India (study B). In study A, subjects were randomized in a 2:1 ratio, and in study B a 1:1 ratio to receive either PsA-TT or PsACWY. Immunogenicity was evaluated by measuring MenA serum bactericidal antibody (SBA) with rabbit complement and by a group A-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay.
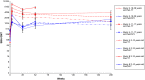
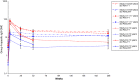
Results: In both studies, substantial SBA decay was observed at 6 months postvaccination in both vaccine groups, although more marked in the PsACWY group. At 1 year and 4 years (only for study A) postvaccination, SBA titers were relatively sustained in the PsA-TT group, whereas a slight increasing trend, more pronounced among the youngest, was observed in the participants aged <18 years in the PsACWY groups. The SBA titers were significantly higher in the PsA-TT group than in the PsACWY group at any time point, and the majority of subjects in the PsA-TT group had SBA titers ≥128 and group A-specific IgG concentrations ≥2 µg/mL at any point in time in both the African and Indian study populations.
Conclusions: Four years after vaccination with a single dose of PsA-TT vaccine in Africa, most subjects are considered protected from MenA disease.
Clinical trials registration: PsA-TT-003 (ISRCTN87739946); PsA-TT-003a (ISRCTN46335400).
Keywords: African meningitis belt; India; MenA conjugate vaccine; antibody persistence.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures
Similar articles
-
Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans.N Engl J Med. 2011 Jun 16;364(24):2293-304. doi: 10.1056/NEJMoa1003812. N Engl J Med. 2011. PMID: 21675889 Clinical Trial.
-
Human Complement Bactericidal Responses to a Group A Meningococcal Conjugate Vaccine in Africans and Comparison to Responses Measured by 2 Other Group A Immunoassays.Clin Infect Dis. 2015 Nov 15;61 Suppl 5:S554-62. doi: 10.1093/cid/civ504. Clin Infect Dis. 2015. PMID: 26553688
-
Influence of Age on Antibody Response and Persistence Following Immunization With MenAfriVac.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S531-9. doi: 10.1093/cid/civ601. Clin Infect Dis. 2015. PMID: 26553685 Free PMC article.
-
Antibody persistence following meningococcal ACWY conjugate vaccine licensed in the European Union by age group and vaccine.Expert Rev Vaccines. 2020 Aug;19(8):745-754. doi: 10.1080/14760584.2020.1800460. Epub 2020 Sep 8. Expert Rev Vaccines. 2020. PMID: 32897762 Review.
-
Ethical Challenges and Lessons Learned During the Clinical Development of a Group A Meningococcal Conjugate Vaccine.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S422-7. doi: 10.1093/cid/civ598. Clin Infect Dis. 2015. PMID: 26553670 Free PMC article. Review.
Cited by
-
Modeling protective meningococcal antibody responses and factors influencing antibody persistence following vaccination with MenAfriVac using machine learning.PLoS One. 2025 May 14;20(5):e0323384. doi: 10.1371/journal.pone.0323384. eCollection 2025. PLoS One. 2025. PMID: 40367245 Free PMC article.
-
Proceedings of the first African Health Forum: effective partnerships and intersectoral collaborations are critical for attainment of Universal Health Coverage in Africa.BMC Proc. 2018 Jul 3;12(Suppl 7):8. doi: 10.1186/s12919-018-0104-2. eCollection 2018. BMC Proc. 2018. PMID: 29997696 Free PMC article.
-
Meningococcal quadrivalent tetanus toxoid conjugate vaccine (MenACWY-TT, Nimenrix™): A review of its immunogenicity, safety, co-administration, and antibody persistence.Hum Vaccin Immunother. 2016 Jul 2;12(7):1825-37. doi: 10.1080/21645515.2016.1143157. Epub 2016 Feb 22. Hum Vaccin Immunother. 2016. PMID: 26900984 Free PMC article. Review.
-
Antibody Persistence 1-5 Years Following Vaccination With MenAfriVac in African Children Vaccinated at 12-23 Months of Age.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S514-20. doi: 10.1093/cid/civ672. Clin Infect Dis. 2015. PMID: 26553683 Free PMC article. Clinical Trial.
-
Community Perspectives Associated With the African PsA-TT (MenAfriVac) Vaccine Trials.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S416-21. doi: 10.1093/cid/civ596. Clin Infect Dis. 2015. PMID: 26553669 Free PMC article. Review.
References
-
- Greenwood B. Editorial: 100 years of epidemic meningitis in West Africa—has anything changed? Trop Med Int Health 2006; 11:773–80. - PubMed
-
- LaForce FM, Ravenscroft N, Djingarey M, Viviani S. Epidemic meningitis due to group A Neisseria meningitidis in the African meningitis belt: a persistent problem with an imminent solution. Vaccine 2009; 27(suppl 2):B13–9. - PubMed
-
- Jodar L, LaForce FM, Ceccarini C, Aguado T, Granoff DM. Meningococcal conjugate vaccine for Africa: a model for development of new vaccines for the poorest countries. Lancet 2003; 361:1902–4. - PubMed
-
- LaForce FM, Konde K, Viviani S, Preziosi MP. The Meningitis Vaccine Project. Vaccine 2007; 25(suppl 1):A97–100. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

