Meningococcal Seroepidemiology 1 Year After the PsA-TT Mass Immunization Campaign in Burkina Faso
- PMID: 26553686
- PMCID: PMC4639492
- DOI: 10.1093/cid/civ519
Meningococcal Seroepidemiology 1 Year After the PsA-TT Mass Immunization Campaign in Burkina Faso
Abstract
Background: A group A meningococcal (MenA) conjugate vaccine, PsA-TT (MenAfriVac), was introduced in Burkina Faso via mass campaigns between September and December 2010, targeting the 1- to 29-year-old population. This study describes specific antibody titers in the general population 11 months later and compares them to preintroduction data obtained during 2008 using the same protocol.
Methods: During October-November 2011, we recruited a representative sample of the population of urban Bobo-Dioulasso aged 6 months to 29 years, who underwent standardized interviews and blood draws. We assessed anti-MenA immunoglobulin G (IgG) concentrations (n = 200) and, using rabbit complement, serum bactericidal antibody (SBA) titers against 2 group A strains: reference strain F8238 (SBAref) (n = 562) and strain 3125 (SBA3125) (n = 200).
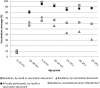
Results: Among the 562 participants, 481 (86%) were aged ≥23 months and had been eligible for the PsA-TT campaign. Among them, vaccine coverage was 86.3% (95% confidence interval [CI], 82.7%-89.9%). Prevalence of putatively protective antibodies among vaccine-eligible age groups was 97.3% (95% CI, 95.9%-98.7%) for SBAref titers ≥128, 83.6% (95% CI, 77.6%-89.7%) for SBA3125 ≥128, and 84.2% (95% CI, 78.7%-89.7%) for anti-MenA IgG ≥2 µg/mL. Compared to the population aged 23 months to 29 years during 2008, geometric mean titers of SBAref were 7.59-fold higher during 2011, 51.88-fold for SBA3125, and 10.56-fold for IgG.
Conclusions: This study shows high seroprevalence against group A meningococci in Burkina Faso following MenAfriVac introduction. Follow-up surveys will provide evidence on the persistence of population-level immunity and the optimal vaccination strategy for long-term control of MenA meningitis in the African meningitis belt.
Keywords: Neisseria meningitidis; conjugate; group A; seroepidemiologic studies; sub-Saharan Africa.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures

References
-
- Sow SO, Okoko BJ, Diallo A et al. . Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. N Engl J Med 2011; 364:2293–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

