Higher Tetanus Toxoid Immunity 2 Years After PsA-TT Introduction in Mali
- PMID: 26553691
- PMCID: PMC4639490
- DOI: 10.1093/cid/civ513
Higher Tetanus Toxoid Immunity 2 Years After PsA-TT Introduction in Mali
Abstract
Background: In 2010, mass vaccination with a then-new meningococcal A polysaccharide-tetanus toxoid protein conjugate vaccine (PsA-TT, or MenAfriVac) was undertaken in 1- to 29-year-olds in Bamako, Mali. Whether vaccination with PsA-TT effectively boosts tetanus immunity in a population with heterogeneous baseline tetanus immunity is not known. We assessed the impact of PsA-TT on tetanus toxoid (TT) immunity by quantifying age- and sex-specific immunity prior to and 2 years after introduction.
Methods: Using a household-based, age-stratified design, we randomly selected participants for a prevaccination serological survey in 2010 and a postvaccination survey in 2012. TT immunoglobulin G (IgG) antibodies were quantified and geometric mean concentrations (GMCs) pre- and postvaccination among all age groups targeted for vaccination were compared. The probability of TT IgG levels ≥0.1 IU/mL (indicating short-term protection) and ≥1.0 IU/mL (indicating long-term protection) by age and sex was determined using logistic regression models.
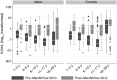
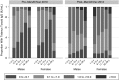
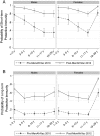
Results: Analysis of 793 prevaccination and 800 postvaccination sera indicated that while GMCs were low pre-PsA-TT, significantly higher GMCs in all age-sex strata were observed 2 years after PsA-TT introduction. The percentage with short-term immunity increased from 57.1% to 88.4% (31.3-point increase; 95% confidence interval [CI], 26.6-36.0;, P < .0001) and with long-term immunity increased from 20.0% to 58.5% (38.5-point increase; 95% CI, 33.7-43.3; P < .0001) pre- and postvaccination.
Conclusions: Significantly higher TT immunity was observed among vaccine-targeted age groups up to 2 years after Mali's PsA-TT mass vaccination campaign. Our results, combined with evidence from clinical trials, strongly suggest that conjugate vaccines containing TT such as PsA-TT should be considered bivalent vaccines because of their ability to boost tetanus immunity.
Keywords: Africa; conjugate; meningococcal vaccines; seroprevalence; tetanus.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures
Similar articles
-
Population-Level Persistence of Immunity 2 Years After the PsA-TT Mass-Vaccination Campaign in Mali.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S547-53. doi: 10.1093/cid/civ602. Clin Infect Dis. 2015. PMID: 26553687 Free PMC article.
-
Neisseria meningitidis Group A IgG1 and IgG2 Subclass Immune Response in African Children Aged 12-23 Months Following Meningococcal Vaccination.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S563-9. doi: 10.1093/cid/civ505. Clin Infect Dis. 2015. PMID: 26553689 Free PMC article. Clinical Trial.
-
MenAfriVac as an Antitetanus Vaccine.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S570-7. doi: 10.1093/cid/civ512. Clin Infect Dis. 2015. PMID: 26553690 Free PMC article. Clinical Trial.
-
MenACWY-TT vaccine for active immunization against invasive meningococcal disease.Expert Rev Vaccines. 2012 May;11(5):523-37. doi: 10.1586/erv.12.32. Expert Rev Vaccines. 2012. PMID: 22827239 Review.
-
MenHibrix: a new combination meningococcal vaccine for infants and toddlers.Ann Pharmacother. 2014 Mar;48(3):404-11. doi: 10.1177/1060028013514375. Epub 2013 Dec 18. Ann Pharmacother. 2014. PMID: 24353263 Review.
Cited by
-
Diphtheria and tetanus seroepidemiology among children in Ukraine, 2017.Vaccine. 2022 Mar 15;40(12):1810-1820. doi: 10.1016/j.vaccine.2022.02.006. Epub 2022 Feb 10. Vaccine. 2022. PMID: 35153095 Free PMC article.
-
Population-Level Persistence of Immunity 2 Years After the PsA-TT Mass-Vaccination Campaign in Mali.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S547-53. doi: 10.1093/cid/civ602. Clin Infect Dis. 2015. PMID: 26553687 Free PMC article.
-
Field investigation of high reported non-neonatal tetanus burden in Uganda, 2016-2017.Int J Epidemiol. 2023 Aug;52(4):1150-1162. doi: 10.1093/ije/dyad005. Epub 2023 Feb 10. Int J Epidemiol. 2023. PMID: 36762894 Free PMC article.
-
Tetanus Immunity among Women Aged 15 to 39 Years in Cambodia: a National Population-Based Serosurvey, 2012.Clin Vaccine Immunol. 2016 Jul 5;23(7):546-54. doi: 10.1128/CVI.00052-16. Print 2016 Jul. Clin Vaccine Immunol. 2016. PMID: 27053629 Free PMC article.
-
Progress towards achieving and maintaining maternal and neonatal tetanus elimination in the African region.Pan Afr Med J. 2017 Jun 22;27(Suppl 3):24. doi: 10.11604/pamj.supp.2017.27.3.11783. eCollection 2017. Pan Afr Med J. 2017. PMID: 29296159 Free PMC article. Review.
References
-
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol 2009; 9:213–20. - PubMed
-
- Frasch CE, Preziosi MP, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac. Hum Vaccin Immunother 2012; 8:715–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

