Modeling Long-term Vaccination Strategies With MenAfriVac in the African Meningitis Belt
- PMID: 26553693
- PMCID: PMC4639487
- DOI: 10.1093/cid/civ508
Modeling Long-term Vaccination Strategies With MenAfriVac in the African Meningitis Belt
Abstract
Background: The introduction of MenAfriVac in campaigns targeting people aged 1-29 years across the African meningitis belt has successfully reduced meningitis incidence and carriage due to Neisseria meningitidis group A (MenA). It is important to consider how best to sustain population protection in the long term.
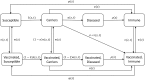
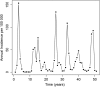
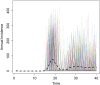
Methods: We created a mathematical model of MenA transmission and disease to investigate the potential impact of a range of immunization strategies. The model is age structured; includes classes of susceptible, carrier, ill, and immune people (who may be vaccinated or unvaccinated); and incorporates seasonal transmission and a stochastic forcing term that models between year variation in rates of transmission. Model parameters were primarily derived from African sources. The model can describe the typical annual incidence of meningitis in the prevaccine era, with irregular epidemics of varying size. Parameter and structural uncertainty were explored in sensitivity analyses.
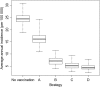
Results: Following MenAfriVac introduction at high uptake, the model predicts excellent short-term disease control. With no subsequent immunization, strong resurgences in disease incidence were predicted after approximately 15 years (assuming 10 years' average vaccine protection). Routine immunization at 9 months of age resulted in lower average annual incidence than regular mass campaigns of 1- to 4-year-olds, provided coverage was above approximately 60%. The strategy with the lowest overall average annual incidence and longest time to resurgence was achieved using a combination strategy of introduction into the Expanded Programme on Immunization at 9 months, 5 years after the initial mass campaigns, with a catch-up targeting unvaccinated 1- to 4-year-olds.
Conclusions: These results can be used to inform policy recommendations for long-term vaccination strategies with MenAfriVac.
Keywords: Africa; mathematical modeling; meningitis; vaccine.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Figures
Similar articles
-
Identifying optimal vaccination strategies for serogroup A Neisseria meningitidis conjugate vaccine in the African meningitis belt.PLoS One. 2013 May 9;8(5):e63605. doi: 10.1371/journal.pone.0063605. Print 2013. PLoS One. 2013. PMID: 23671685 Free PMC article.
-
Response thresholds for epidemic meningitis in sub-Saharan Africa following the introduction of MenAfriVac®.Vaccine. 2015 Nov 17;33(46):6212-7. doi: 10.1016/j.vaccine.2015.09.107. Epub 2015 Oct 14. Vaccine. 2015. PMID: 26463444
-
Antibody kinetics following vaccination with MenAfriVac: an analysis of serological data from randomised trials.Lancet Infect Dis. 2019 Mar;19(3):327-336. doi: 10.1016/S1473-3099(18)30674-1. Epub 2019 Feb 10. Lancet Infect Dis. 2019. PMID: 30745277
-
Meningococcal carriage by age in the African meningitis belt: a systematic review and meta-analysis.Epidemiol Infect. 2019 Jan;147:e228. doi: 10.1017/S0950268819001134. Epidemiol Infect. 2019. PMID: 31364554 Free PMC article.
-
From Epidemic Meningitis Vaccines for Africa to the Meningitis Vaccine Project.Clin Infect Dis. 2015 Nov 15;61 Suppl 5(Suppl 5):S391-5. doi: 10.1093/cid/civ593. Clin Infect Dis. 2015. PMID: 26553665 Free PMC article. Review.
Cited by
-
Successful African introduction of a new Group A meningococcal conjugate vaccine: Future challenges and next steps.Hum Vaccin Immunother. 2018 May 4;14(5):1098-1102. doi: 10.1080/21645515.2017.1378841. Epub 2017 Nov 8. Hum Vaccin Immunother. 2018. PMID: 28968148 Free PMC article.
-
Serogroup-specific meningococcal carriage by age group: a systematic review and meta-analysis.BMJ Open. 2019 Apr 20;9(4):e024343. doi: 10.1136/bmjopen-2018-024343. BMJ Open. 2019. PMID: 31005910 Free PMC article.
-
Impact of serogroup A meningococcal conjugate vaccine for Africa.Hum Vaccin Immunother. 2018 May 4;14(5):1116-1117. doi: 10.1080/21645515.2017.1412022. Epub 2018 Jan 16. Hum Vaccin Immunother. 2018. PMID: 29194010 Free PMC article.
-
Disease transmission and mass gatherings: a case study on meningococcal infection during Hajj.BMC Infect Dis. 2022 Mar 22;22(1):275. doi: 10.1186/s12879-022-07234-4. BMC Infect Dis. 2022. PMID: 35317742 Free PMC article.
-
Kinetics of maternally-derived serogroup A, C, Y and W-specific meningococcal immunoglobulin G in Malian women and infants.Vaccine. 2019 Apr 24;37(18):2477-2481. doi: 10.1016/j.vaccine.2019.03.045. Epub 2019 Apr 2. Vaccine. 2019. PMID: 30952500 Free PMC article. Clinical Trial.
References
-
- Frasch CE, Preziosi MP, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac(TM). Hum Vaccin Immunother 2012; 8:715–24. - PubMed
-
- Kristiansen PA, Diomande F, Ba AK et al. . Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis 2013; 56:354–63. - PubMed
-
- Trotter CL, McVernon J, Ramsay ME et al. . Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine 2008; 26:4434–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

