New genetic regulators question relevance of abundant yolk protein production in C. elegans
- PMID: 26553710
- PMCID: PMC4639837
- DOI: 10.1038/srep16381
New genetic regulators question relevance of abundant yolk protein production in C. elegans
Abstract
Vitellogenesis or maternal yolk formation is considered critical to the reproduction of egg-laying animals. In invertebrates, however, most of its regulatory genes are still unknown. Via a combined mapping and whole-genome sequencing strategy, we performed a forward genetic screen to isolate novel regulators of yolk production in the nematode model system Caenorhabditis elegans. In addition to isolating new alleles of rab-35, rab-10 and M04F3.2, we identified five mutant alleles corresponding to three novel regulatory genes potently suppressing the expression of a GFP-based yolk reporter. We confirmed that mutations in vrp-1, ceh-60 and lrp-2 disrupt endogenous yolk protein synthesis at the transcriptional and translational level. In contrast to current beliefs, our discovered set of mutants with strongly reduced yolk proteins did not show serious reproduction defects. This raises questions as to whether yolk proteins per se are needed for ultimate reproductive success.
Figures
 ), vit-2::gfp reporter (
), vit-2::gfp reporter ( ) and tam-1(lst538) (
) and tam-1(lst538) ( ) controls, endogenous YP170 levels are considerably lower in all YPR mutant populations, i.e. vrp-1(lst461) (
) controls, endogenous YP170 levels are considerably lower in all YPR mutant populations, i.e. vrp-1(lst461) ( ), vrp-1(lst539) (
), vrp-1(lst539) ( ), ceh-60(lst466) (
), ceh-60(lst466) ( ), ceh-60(lst491) (
), ceh-60(lst491) ( ), lrp-2(lst464) (
), lrp-2(lst464) ( ) and lrp-2(gk556942) (
) and lrp-2(gk556942) ( ). Also in a wild-type background, mutant YPR alleles suppress endogenous YP170 (
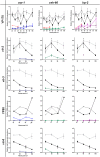
). Also in a wild-type background, mutant YPR alleles suppress endogenous YP170 ( ). The vit-2 (second row) and vit-3 (third row) mRNA expression profiles are consistent with these YP170 yolk protein data (first row). YP88 immunoblot data are expressed relative to each sample’s total protein signal (fourth row; Supplementary Table S2). In sharp contrast to both ceh-60, the vrp-1(lst461) and both lrp-2 mutants, the vrp-1(lst539) mutant still displays some YP88 immunoreactivity. The underlying vit-6 (bottom row) mRNA expression profiles again correlate well with these YP88 yolk protein data (Supplementary Table S2). For each indicated time point throughout reproductive development, the mean value of a maximum of three (mRNA) or four (protein) biologically independent measurements ± SEM is plotted and connected to assist in overall profile evaluation (see also Supplementary Table S2).
). The vit-2 (second row) and vit-3 (third row) mRNA expression profiles are consistent with these YP170 yolk protein data (first row). YP88 immunoblot data are expressed relative to each sample’s total protein signal (fourth row; Supplementary Table S2). In sharp contrast to both ceh-60, the vrp-1(lst461) and both lrp-2 mutants, the vrp-1(lst539) mutant still displays some YP88 immunoreactivity. The underlying vit-6 (bottom row) mRNA expression profiles again correlate well with these YP88 yolk protein data (Supplementary Table S2). For each indicated time point throughout reproductive development, the mean value of a maximum of three (mRNA) or four (protein) biologically independent measurements ± SEM is plotted and connected to assist in overall profile evaluation (see also Supplementary Table S2).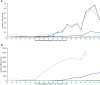
 ), vit-2::gfp reporter control (
), vit-2::gfp reporter control ( ), vrp-1(lst461) (
), vrp-1(lst461) ( ), ceh-60(lst466) (
), ceh-60(lst466) ( ), all quantified as of the beginning of the L4 stage (profiles connect single measurements). (a) vit-2 mRNA levels of wild-type and vit-2::gfp reporter animals are heavily up-regulated upon becoming young adults, whereas this is not convincingly so in vrp-1(lst461) and nearly not at all in ceh-60(lst466) mutants. The vit-2 mRNA pool in all except wild-type animals also contains mRNA derived from the translational vit-2::gfp reporter construct. (b) In wild type, vit-6 up-regulation initiates slightly before that of vit-2, and covers an impressively larger dynamic range. vit-6 is the only YP88/YP115-providing gene, whereas the other five C. elegans vit genes can contribute to the YP170 pool. Also here, a very moderate (vrp-1(lst461)) - to no (ceh-60(lst466)) vit-6 up-regulation is observed in the selected YPR mutants. The lin-42 profile in panel b has been multiplied by 40 as compared to all other figures in this manuscript to facilitate visibility.
), all quantified as of the beginning of the L4 stage (profiles connect single measurements). (a) vit-2 mRNA levels of wild-type and vit-2::gfp reporter animals are heavily up-regulated upon becoming young adults, whereas this is not convincingly so in vrp-1(lst461) and nearly not at all in ceh-60(lst466) mutants. The vit-2 mRNA pool in all except wild-type animals also contains mRNA derived from the translational vit-2::gfp reporter construct. (b) In wild type, vit-6 up-regulation initiates slightly before that of vit-2, and covers an impressively larger dynamic range. vit-6 is the only YP88/YP115-providing gene, whereas the other five C. elegans vit genes can contribute to the YP170 pool. Also here, a very moderate (vrp-1(lst461)) - to no (ceh-60(lst466)) vit-6 up-regulation is observed in the selected YPR mutants. The lin-42 profile in panel b has been multiplied by 40 as compared to all other figures in this manuscript to facilitate visibility.
 ), vit-2::gfp (
), vit-2::gfp ( ), vrp-1(lst461) (
), vrp-1(lst461) ( ) and ceh-60(lst491) (
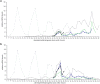
) and ceh-60(lst491) ( ) (all profiles connect single measurements). In line with their effects on yolk levels in C. elegans, YPR genes are up-regulated in the later part of the developmental cycle (also Supplementary Fig. S5). vrp-1 levels are affected in ceh-60 mutants and vice-versa.
) (all profiles connect single measurements). In line with their effects on yolk levels in C. elegans, YPR genes are up-regulated in the later part of the developmental cycle (also Supplementary Fig. S5). vrp-1 levels are affected in ceh-60 mutants and vice-versa.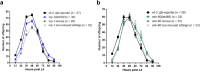

 ) individuals cannot cope with survival in absence of food (p = 1.7E-09) (also Supplementary Fig. S7). While also declining slightly earlier, tam-1(lst538) (
) individuals cannot cope with survival in absence of food (p = 1.7E-09) (also Supplementary Fig. S7). While also declining slightly earlier, tam-1(lst538) ( ) controls do not differ significantly from wild-type decline (p = 0.669). (b) Also vrp-1(lst539) (
) controls do not differ significantly from wild-type decline (p = 0.669). (b) Also vrp-1(lst539) ( ) shows a decrease in fitness under these conditions (p = 8.68E-05), though far less outspoken than the ceh-60(lst466) mutant. Because L1 diapause survival depends on culture density, panels (a) with 11 worms/μl and (b) with 18 worms/μl cannot be directly compared.
) shows a decrease in fitness under these conditions (p = 8.68E-05), though far less outspoken than the ceh-60(lst466) mutant. Because L1 diapause survival depends on culture density, panels (a) with 11 worms/μl and (b) with 18 worms/μl cannot be directly compared.References
-
- Tata J. R., James T. C., Watson C. S., Williams J. L. & Wolffe A. P. Hormonal regulation and expression of vitellogenin multigene family. Ciba Found. Symp. 98, 96–110 (1983). - PubMed
-
- Awruch C. A. Reproductive endocrinology in chondrichthyans: The present and the future. Gen. Comp. Endocrinol. 192, 60–70 (2013). - PubMed
-
- Sharrock W. J. Cleavage of two yolk proteins from a precursor in Caenorhabditis elegans. J. Mol. Biol. 174, 419–431 (1984). - PubMed
-
- Winter C. In Reproductive biology of invertebrates - Progress in vitellogenesis (A. Raikhel & T. Sappington) 1–27 (Plenum Press, 2002).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

