The Circadian Clock Gene Period1 Connects the Molecular Clock to Neural Activity in the Suprachiasmatic Nucleus
- PMID: 26553726
- PMCID: PMC4710129
- DOI: 10.1177/1759091415610761
The Circadian Clock Gene Period1 Connects the Molecular Clock to Neural Activity in the Suprachiasmatic Nucleus
Abstract
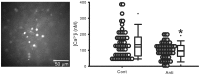
The neural activity patterns of suprachiasmatic nucleus (SCN) neurons are dynamically regulated throughout the circadian cycle with highest levels of spontaneous action potentials during the day. These rhythms in electrical activity are critical for the function of the circadian timing system and yet the mechanisms by which the molecular clockwork drives changes in the membrane are not well understood. In this study, we sought to examine how the clock gene Period1 (Per1) regulates the electrical activity in the mouse SCN by transiently and selectively decreasing levels of PER1 through use of an antisense oligodeoxynucleotide. We found that this treatment effectively reduced SCN neural activity. Direct current injection to restore the normal membrane potential partially, but not completely, returned firing rate to normal levels. The antisense treatment also reduced baseline [Ca(2+)]i levels as measured by Fura2 imaging technique. Whole cell patch clamp recording techniques were used to examine which specific potassium currents were altered by the treatment. These recordings revealed that the large conductance [Ca(2+)]i-activated potassium currents were reduced in antisense-treated neurons and that blocking this current mimicked the effects of the anti-sense on SCN firing rate. These results indicate that the circadian clock gene Per1 alters firing rate in SCN neurons and raise the possibility that the large conductance [Ca(2+)]i-activated channel is one of the targets.
Keywords: BK currents; Per1; calcium; circadian.
© The Author(s) 2015.
Figures



References
-
- Akiyama M., Kouzu Y., Takahashi S., Wakamatsu H., Moriya T., Maetani M., Shibata S. (1999) Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 19: 1115–1121. - PMC - PubMed
-
- Albus H., Bonnefont X., Chaves I., Yasui A., Doczy J., van der Horst G. T., Meijer J. H. (2002) Cryptochrome-deficient mice lack circadian electrical activity in the suprachiasmatic nuclei. Current Biology: CB 12: 1130–1133. - PubMed
-
- Belle M. D., Diekman C. O., Forger D. B., Piggins H. D. (2009) Daily electrical silencing in the mammalian circadian clock. Science 326: 281–284. - PubMed
-
- Brown S. A., Kowalska E., Dallmann R. (2012) (Re)inventing the circadian feedback loop. Developmental Cell 22: 477–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

