Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts
- PMID: 26553810
- PMCID: PMC4797265
- DOI: 10.1093/nar/gkv1210
Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts
Abstract
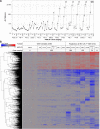
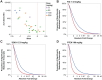
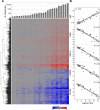
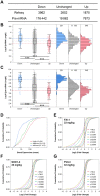
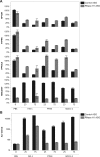
High affinity antisense oligonucleotides (ASOs) containing bicylic modifications (BNA) such as locked nucleic acid (LNA) designed to induce target RNA cleavage have been shown to have enhanced potency along with a higher propensity to cause hepatotoxicity. In order to understand the mechanism of this hepatotoxicity, transcriptional profiles were collected from the livers of mice treated with a panel of highly efficacious hepatotoxic or non-hepatotoxic LNA ASOs. We observed highly selective transcript knockdown in mice treated with non-hepatotoxic LNA ASOs, while the levels of many unintended transcripts were reduced in mice treated with hepatotoxic LNA ASOs. This transcriptional signature was concurrent with on-target RNA reduction and preceded transaminitis. Remarkably, the mRNA transcripts commonly reduced by toxic LNA ASOs were generally not strongly associated with any particular biological process, cellular component or functional group. However, they tended to have much longer pre-mRNA transcripts. We also demonstrate that the off-target RNA knockdown and hepatotoxicity is attenuated by RNase H1 knockdown, and that this effect can be generalized to high affinity modifications beyond LNA. This suggests that for a certain set of ASOs containing high affinity modifications such as LNA, hepatotoxicity can occur as a result of unintended off-target RNase H1 dependent RNA degradation.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
References
-
- Yacyshyn B., Bowen-Yacyshyn M.B., Shanahan W. The clinical experience of antisense therapy to ICAM-1 in Crohn's disease. Curr. Opin. Mol. Ther. 1999;1:332–335. - PubMed
-
- Bennett F.C. In: Antisense Drug Technologies, Second Edition. Crooke S.T., editor. Vol. 2. LLC, Boca Raton: Taylor & Francis Group; 2007. pp. 273–303. Vol.
-
- Monia B.P., Lesnik E.A., Gonzalez C., Lima W.F., McGee D., Guinosso C.J., Kawasaki A.M., Cook P.D., Freier S.M. Evaluation of 2′ modified oligonucleotides containing deoxy gaps as antisense inhibitors of gene expression. J. Biol. Chem. 1993;268:14514–14522. - PubMed
-
- Crooke S.T., Vickers T., Lima W., Wu H. In: Antisense drug technology, principles, strategies and applications. Crooke S.T., editor. Vol. 2. Boca Raton, FL: Taylor & Francis Group; 2007. pp. 5–46. Vol.
-
- Bennett C.F., Swayze E.E. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

