Conformational signaling required for synaptic plasticity by the NMDA receptor complex
- PMID: 26553983
- PMCID: PMC4664326
- DOI: 10.1073/pnas.1520029112
Conformational signaling required for synaptic plasticity by the NMDA receptor complex
Abstract
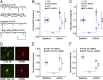
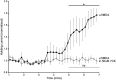
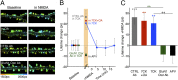
The NMDA receptor (NMDAR) is known to transmit important information by conducting calcium ions. However, some recent studies suggest that activation of NMDARs can trigger synaptic plasticity in the absence of ion flow. Does ligand binding transmit information to signaling molecules that mediate synaptic plasticity? Using Förster resonance energy transfer (FRET) imaging of fluorescently tagged proteins expressed in neurons, conformational signaling is identified within the NMDAR complex that is essential for downstream actions. Ligand binding transiently reduces FRET between the NMDAR cytoplasmic domain (cd) and the associated protein phosphatase 1 (PP1), requiring NMDARcd movement, and persistently reduces FRET between the NMDARcd and calcium/calmodulin-dependent protein kinase II (CaMKII), a process requiring PP1 activity. These studies directly monitor agonist-driven conformational signaling at the NMDAR complex required for synaptic plasticity.
Keywords: LTD; NMDAR-CaMKII interaction; NMDAR-PP1 FRET; ion-flow independent; long-term depression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

