Direct measurements of the coordination of lever arm swing and the catalytic cycle in myosin V
- PMID: 26553992
- PMCID: PMC4664342
- DOI: 10.1073/pnas.1517566112
Direct measurements of the coordination of lever arm swing and the catalytic cycle in myosin V
Abstract
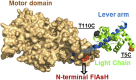
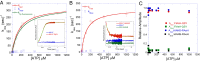
Myosins use a conserved structural mechanism to convert the energy from ATP hydrolysis into a large swing of the force-generating lever arm. The precise timing of the lever arm movement with respect to the steps in the actomyosin ATPase cycle has not been determined. We have developed a FRET system in myosin V that uses three donor-acceptor pairs to examine the kinetics of lever arm swing during the recovery and power stroke phases of the ATPase cycle. During the recovery stroke the lever arm swing is tightly coupled to priming the active site for ATP hydrolysis. The lever arm swing during the power stroke occurs in two steps, a fast step that occurs before phosphate release and a slow step that occurs before ADP release. Time-resolved FRET demonstrates a 20-Å change in distance between the pre- and postpower stroke states and shows that the lever arm is more dynamic in the postpower stroke state. Our results suggest myosin binding to actin in the ADP.Pi complex triggers a rapid power stroke that gates the release of phosphate, whereas a second slower power stroke may be important for mediating strain sensitivity.
Keywords: FRET; actin; force generation; kinetics; myosin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
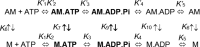
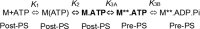

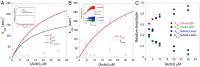
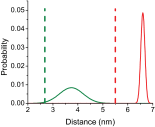
References
-
- Sellers JR. Myosins: A diverse superfamily. Biochim Biophys Acta. 2000;1496(1):3–22. - PubMed
-
- Sweeney HL, Houdusse A. Structural and functional insights into the Myosin motor mechanism. Annu Rev Biophys. 2010;39:539–557. - PubMed
-
- Huxley HE. Sliding filaments and molecular motile systems. J Biol Chem. 1990;265(15):8347–8350. - PubMed
-
- Holmes KC. The swinging lever-arm hypothesis of muscle contraction. Curr Biol. 1997;7(2):R112–R118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

