Directed evolution of the tryptophan synthase β-subunit for stand-alone function recapitulates allosteric activation
- PMID: 26553994
- PMCID: PMC4664345
- DOI: 10.1073/pnas.1516401112
Directed evolution of the tryptophan synthase β-subunit for stand-alone function recapitulates allosteric activation
Abstract
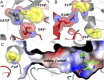
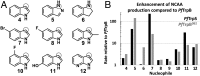
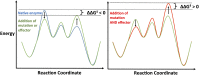
Enzymes in heteromeric, allosterically regulated complexes catalyze a rich array of chemical reactions. Separating the subunits of such complexes, however, often severely attenuates their catalytic activities, because they can no longer be activated by their protein partners. We used directed evolution to explore allosteric regulation as a source of latent catalytic potential using the β-subunit of tryptophan synthase from Pyrococcus furiosus (PfTrpB). As part of its native αββα complex, TrpB efficiently produces tryptophan and tryptophan analogs; activity drops considerably when it is used as a stand-alone catalyst without the α-subunit. Kinetic, spectroscopic, and X-ray crystallographic data show that this lost activity can be recovered by mutations that reproduce the effects of complexation with the α-subunit. The engineered PfTrpB is a powerful platform for production of Trp analogs and for further directed evolution to expand substrate and reaction scope.
Keywords: PLP; allostery; noncanonical amino acid; protein engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A Panel of TrpB Biocatalysts Derived from Tryptophan Synthase through the Transfer of Mutations that Mimic Allosteric Activation.Angew Chem Int Ed Engl. 2016 Sep 12;55(38):11577-81. doi: 10.1002/anie.201606242. Epub 2016 Aug 11. Angew Chem Int Ed Engl. 2016. PMID: 27510733 Free PMC article.
-
Directed Evolution Mimics Allosteric Activation by Stepwise Tuning of the Conformational Ensemble.J Am Chem Soc. 2018 Jun 13;140(23):7256-7266. doi: 10.1021/jacs.8b03490. Epub 2018 May 17. J Am Chem Soc. 2018. PMID: 29712420 Free PMC article.
-
Engineered Biosynthesis of β-Alkyl Tryptophan Analogues.Angew Chem Int Ed Engl. 2018 Nov 5;57(45):14764-14768. doi: 10.1002/anie.201807998. Epub 2018 Oct 12. Angew Chem Int Ed Engl. 2018. PMID: 30215880
-
Tryptophan Synthase: Biocatalyst Extraordinaire.Chembiochem. 2021 Jan 5;22(1):5-16. doi: 10.1002/cbic.202000379. Epub 2020 Sep 22. Chembiochem. 2021. PMID: 32677310 Free PMC article. Review.
-
Tryptophan synthase: the workings of a channeling nanomachine.Trends Biochem Sci. 2008 Jun;33(6):254-64. doi: 10.1016/j.tibs.2008.04.008. Epub 2008 May 15. Trends Biochem Sci. 2008. PMID: 18486479 Review.
Cited by
-
Allosteric Activation Shifts the Rate-Limiting Step in a Short-Form ATP Phosphoribosyltransferase.Biochemistry. 2018 Jul 24;57(29):4357-4367. doi: 10.1021/acs.biochem.8b00559. Epub 2018 Jul 10. Biochemistry. 2018. PMID: 29940105 Free PMC article.
-
Modular control of l-tryptophan isotopic substitution via an efficient biosynthetic cascade.Org Biomol Chem. 2020 Jun 10;18(22):4189-4192. doi: 10.1039/d0ob00868k. Org Biomol Chem. 2020. PMID: 32452506 Free PMC article.
-
Human gut-associated Bifidobacterium species salvage exogenous indole, a uremic toxin precursor, to synthesize indole-3-lactic acid via tryptophan.Gut Microbes. 2024 Jan-Dec;16(1):2347728. doi: 10.1080/19490976.2024.2347728. Epub 2024 May 5. Gut Microbes. 2024. PMID: 38706226 Free PMC article.
-
Molecular Basis for Cγ-N Bond Formation by PLP-Dependent Enzyme LolC.Biochemistry. 2024 Dec 17;63(24):3348-3356. doi: 10.1021/acs.biochem.4c00588. Epub 2024 Dec 6. Biochemistry. 2024. PMID: 39641520 Free PMC article.
-
Resistance of Mycobacterium tuberculosis to indole 4-carboxamides occurs through alterations in drug metabolism and tryptophan biosynthesis.Cell Chem Biol. 2021 Aug 19;28(8):1180-1191.e20. doi: 10.1016/j.chembiol.2021.02.023. Epub 2021 Mar 24. Cell Chem Biol. 2021. PMID: 33765439 Free PMC article.
References
-
- Du L, Lou L. PKS and NRPS release mechanisms. Nat Prod Rep. 2010;27(2):255–278. - PubMed
-
- Hyde CC, Ahmed SA, Padlan EA, Miles EW, Davies DR. Three-dimensional structure of the tryptophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium. J Biol Chem. 1988;263(33):17857–17871. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

