Unmyelinated type II afferent neurons report cochlear damage
- PMID: 26553995
- PMCID: PMC4664349
- DOI: 10.1073/pnas.1515228112
Unmyelinated type II afferent neurons report cochlear damage
Abstract
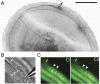
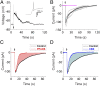
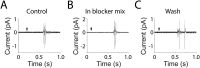
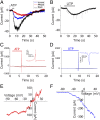
In the mammalian cochlea, acoustic information is carried to the brain by the predominant (95%) large-diameter, myelinated type I afferents, each of which is postsynaptic to a single inner hair cell. The remaining thin, unmyelinated type II afferents extend hundreds of microns along the cochlear duct to contact many outer hair cells. Despite this extensive arbor, type II afferents are weakly activated by outer hair cell transmitter release and are insensitive to sound. Intriguingly, type II afferents remain intact in damaged regions of the cochlea. Here, we show that type II afferents are activated when outer hair cells are damaged. This response depends on both ionotropic (P2X) and metabotropic (P2Y) purinergic receptors, binding ATP released from nearby supporting cells in response to hair cell damage. Selective activation of P2Y receptors increased type II afferent excitability by the closure of KCNQ-type potassium channels, a potential mechanism for the painful hypersensitivity (that we term "noxacusis" to distinguish from hyperacusis without pain) that can accompany hearing loss. Exposure to the KCNQ channel activator retigabine suppressed the type II fiber's response to hair cell damage. Type II afferents may be the cochlea's nociceptors, prompting avoidance of further damage to the irreparable inner ear.
Keywords: ATP; acoustic trauma; hyperacusis; noxacusis; type II cochlear afferents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

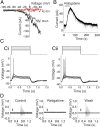
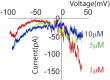
References
-
- Brask T. The noise protection effect of the stapedius reflex. Acta Otolaryngol Suppl. 1979;360:116–117. - PubMed
-
- Guinan JJ., Jr Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27(6):589–607. - PubMed
-
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol. 2005;125(5):503–509. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

