A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis
- PMID: 26554016
- PMCID: PMC4664319
- DOI: 10.1073/pnas.1514598112
A vacuolar phosphate transporter essential for phosphate homeostasis in Arabidopsis
Abstract
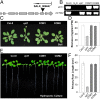
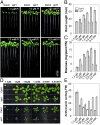
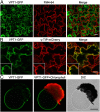
Inorganic phosphate (Pi) is stored in the vacuole, allowing plants to adapt to variable Pi availability in the soil. The transporters that mediate Pi sequestration into vacuole remain unknown, however. Here we report the functional characterization of Vacuolar Phosphate Transporter 1 (VPT1), an SPX domain protein that transports Pi into the vacuole in Arabidopsis. The vpt1 mutant plants were stunted and consistently retained less Pi than wild type plants, especially when grown in medium containing high levels of Pi. In seedlings, VPT1 was expressed primarily in younger tissues under normal conditions, but was strongly induced by high-Pi conditions in older tissues, suggesting that VPT1 functions in Pi storage in young tissues and in detoxification of high Pi in older tissues. As a result, disruption of VPT1 rendered plants hypersensitive to both low-Pi and high-Pi conditions, reducing the adaptability of plants to changing Pi availability. Patch-clamp analysis of isolated vacuoles showed that the Pi influx current was severely reduced in vpt1 compared with wild type plants. When ectopically expressed in Nicotiana benthamiana mesophyll cells, VPT1 mediates vacuolar influx of anions, including Pi, SO4(2-), NO3(-), Cl(-), and malate with Pi as that preferred anion. The VPT1-mediated Pi current amplitude was dependent on cytosolic phosphate concentration. Single-channel analysis showed that the open probability of VPT1 was increased with the increase in transtonoplast potential. We conclude that VPT1 is a transporter responsible for vacuolar Pi storage and is essential for Pi adaptation in Arabidopsis.
Keywords: anion channel; patch clamp; phosphorus nutrition; vacuolar phosphate sequestration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Vacuolar Phosphate Transporters Contribute to Systemic Phosphate Homeostasis Vital for Reproductive Development in Arabidopsis.Plant Physiol. 2019 Feb;179(2):640-655. doi: 10.1104/pp.18.01424. Epub 2018 Dec 14. Plant Physiol. 2019. PMID: 30552198 Free PMC article.
-
Vacuolar SPX-MFS transporters are essential for phosphate adaptation in plants.Plant Signal Behav. 2016 Aug 2;11(8):e1213474. doi: 10.1080/15592324.2016.1213474. Plant Signal Behav. 2016. PMID: 27467463 Free PMC article.
-
Vacuolar Phosphate Transporter 1 (VPT1) Affects Arsenate Tolerance by Regulating Phosphate Homeostasis in Arabidopsis.Plant Cell Physiol. 2018 Jul 1;59(7):1345-1352. doi: 10.1093/pcp/pcy025. Plant Cell Physiol. 2018. PMID: 29420798
-
Role of vacuoles in phosphorus storage and remobilization.J Exp Bot. 2017 Jun 1;68(12):3045-3055. doi: 10.1093/jxb/erw481. J Exp Bot. 2017. PMID: 28077447 Review.
-
SPX proteins regulate Pi homeostasis and signaling in different subcellular level.Plant Signal Behav. 2015;10(9):e1061163. doi: 10.1080/15592324.2015.1061163. Plant Signal Behav. 2015. PMID: 26224365 Free PMC article. Review.
Cited by
-
Identification of plant vacuolar transporters mediating phosphate storage.Nat Commun. 2016 Mar 31;7:11095. doi: 10.1038/ncomms11095. Nat Commun. 2016. PMID: 27029856 Free PMC article.
-
Genetically controlling VACUOLAR PHOSPHATE TRANSPORTER 1 contributes to low-phosphorus seeds in Arabidopsis.Plant Signal Behav. 2023 Dec 31;18(1):2186641. doi: 10.1080/15592324.2023.2186641. Plant Signal Behav. 2023. PMID: 36890723 Free PMC article.
-
Phosphorus uptake, transport, and signaling in woody and model plants.For Res (Fayettev). 2024 May 6;4:e017. doi: 10.48130/forres-0024-0014. eCollection 2024. For Res (Fayettev). 2024. PMID: 39524430 Free PMC article. Review.
-
Structural mechanism underlying PHO1;H1-mediated phosphate transport in Arabidopsis.Nat Plants. 2025 Feb;11(2):309-320. doi: 10.1038/s41477-024-01895-6. Epub 2025 Jan 21. Nat Plants. 2025. PMID: 39838070
-
The transcription factor OsWRKY10 inhibits phosphate uptake via suppressing OsPHT1;2 expression under phosphate-replete conditions in rice.J Exp Bot. 2023 Feb 5;74(3):1074-1089. doi: 10.1093/jxb/erac456. J Exp Bot. 2023. PMID: 36402551 Free PMC article.
References
-
- Shin H, Shin HS, Dewbre GR, Harrison MJ. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004;39(4):629–642. - PubMed
-
- Ai P, et al. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009;57(5):798–809. - PubMed
-
- Shane MW, McCully ME, Lambers H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae) J Exp Bot. 2004;55(399):1033–1044. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

