Leukocyte-specific protein 1 regulates T-cell migration in rheumatoid arthritis
- PMID: 26554018
- PMCID: PMC4664344
- DOI: 10.1073/pnas.1514152112
Leukocyte-specific protein 1 regulates T-cell migration in rheumatoid arthritis
Abstract
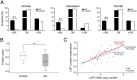
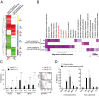
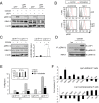
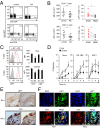
Copy number variations (CNVs) have been implicated in human diseases. However, it remains unclear how they affect immune dysfunction and autoimmune diseases, including rheumatoid arthritis (RA). Here, we identified a novel leukocyte-specific protein 1 (LSP1) deletion variant for RA susceptibility located in 11p15.5. We replicated that the copy number of LSP1 gene is significantly lower in patients with RA, which correlates positively with LSP1 protein expression levels. Differentially expressed genes in Lsp1-deficient primary T cells represent cell motility and immune and cytokine responses. Functional assays demonstrated that LSP1, induced by T-cell receptor activation, negatively regulates T-cell migration by reducing ERK activation in vitro. In mice with T-cell-dependent chronic inflammation, loss of Lsp1 promotes migration of T cells into the target tissues as well as draining lymph nodes, exacerbating disease severity. Moreover, patients with RA show diminished expression of LSP1 in peripheral T cells with increased migratory capacity, suggesting that the defect in LSP1 signaling lowers the threshold for T-cell activation. To our knowledge, our work is the first to demonstrate how CNVs result in immune dysfunction and a disease phenotype. Particularly, our data highlight the importance of LSP1 CNVs and LSP1 insufficiency in the pathogenesis of RA and provide previously unidentified insights into the mechanisms underlying T-cell migration toward the inflamed synovium in RA.
Keywords: T-cell function; cell migration; copy number variation; leukocyte-specific protein 1; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Franz CM, Jones GE, Ridley AJ. Cell migration in development and disease. Dev Cell. 2002;2(2):153–158. - PubMed
-
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: Present and future therapeutic targets. Nat Immunol. 2005;6(12):1182–1190. - PubMed
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

