Global shape mimicry of tRNA within a viral internal ribosome entry site mediates translational reading frame selection
- PMID: 26554019
- PMCID: PMC4664324
- DOI: 10.1073/pnas.1512088112
Global shape mimicry of tRNA within a viral internal ribosome entry site mediates translational reading frame selection
Abstract
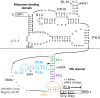
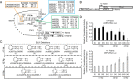
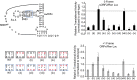
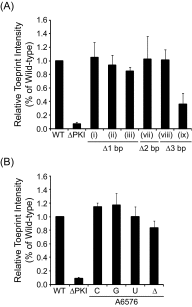
The dicistrovirus intergenic region internal ribosome entry site (IRES) adopts a triple-pseudoknotted RNA structure and occupies the core ribosomal E, P, and A sites to directly recruit the ribosome and initiate translation at a non-AUG codon. A subset of dicistrovirus IRESs directs translation in the 0 and +1 frames to produce the viral structural proteins and a +1 overlapping open reading frame called ORFx, respectively. Here we show that specific mutations of two unpaired adenosines located at the core of the three-helical junction of the honey bee dicistrovirus Israeli acute paralysis virus (IAPV) IRES PKI domain can uncouple 0 and +1 frame translation, suggesting that the structure adopts distinct conformations that contribute to 0 or +1 frame translation. Using a reconstituted translation system, we show that ribosomes assembled on mutant IRESs that direct exclusive 0 or +1 frame translation lack reading frame fidelity. Finally, a nuclear magnetic resonance/small-angle X-ray scattering hybrid approach reveals that the PKI domain of the IAPV IRES adopts an RNA structure that resembles a complete tRNA. The tRNA shape-mimicry enables the viral IRES to gain access to the ribosome tRNA-binding sites and form intermolecular contacts with the ribosome that are necessary for initiating IRES translation in a specific reading frame.
Keywords: RNA; internal ribosome entry site; ribosome; translation; virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 2001;15(13):1593–1612. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- S10RR025062/RR/NCRR NIH HHS/United States
- S10 RR027000/RR/NCRR NIH HHS/United States
- S10RR029220/RR/NCRR NIH HHS/United States
- S10 RR025062/RR/NCRR NIH HHS/United States
- R01 GM072447/GM/NIGMS NIH HHS/United States
- S10RR027000/RR/NCRR NIH HHS/United States
- S10 RR029220/RR/NCRR NIH HHS/United States
- P41GM103399/GM/NIGMS NIH HHS/United States
- S10 RR002781/RR/NCRR NIH HHS/United States
- P41 GM103399/GM/NIGMS NIH HHS/United States
- S10 RR008438/RR/NCRR NIH HHS/United States
- MOP-81244/Canadian Institutes of Health Research/Canada
- S10 RR023438/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

