Vertebrate brains and evolutionary connectomics: on the origins of the mammalian 'neocortex'
- PMID: 26554047
- PMCID: PMC4650131
- DOI: 10.1098/rstb.2015.0060
Vertebrate brains and evolutionary connectomics: on the origins of the mammalian 'neocortex'
Abstract
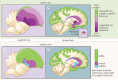
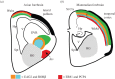
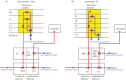
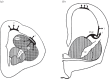
The organization of the non-mammalian forebrain had long puzzled neurobiologists. Unlike typical mammalian brains, the telencephalon is not organized in a laminated 'cortical' manner, with distinct cortical areas dedicated to individual sensory modalities or motor functions. The two major regions of the telencephalon, the basal ventricular ridge (BVR) and the dorsal ventricular ridge (DVR), were loosely referred to as being akin to the mammalian basal ganglia. The telencephalon of non-mammalian vertebrates appears to consist of multiple 'subcortical' groups of cells. Analysis of the nuclear organization of the avian brain, its connections, molecular properties and physiology, and organization of its pattern of circuitry and function relative to that of mammals, collectively referred to as 'evolutionary connectomics', revealed that only a restricted portion of the BVR is homologous to the basal ganglia of mammals. The remaining dorsal regions of the DVR, wulst and arcopallium of the avian brain contain telencephalic inputs and outputs remarkably similar to those of the individual layers of the mammalian 'neocortex', hippocampus and amygdala, with instances of internuclear connections strikingly similar to those found between cortical layers and within radial 'columns' in the mammalian sensory and motor cortices. The molecular properties of these 'nuclei' in birds and reptiles are similar to those of the corresponding layers of the mammalian neocortex. The fundamental pathways and cell groups of the auditory, visual and somatosensory systems of the thalamus and telencephalon are homologous at the cellular, circuit, network and gene levels, and are of great antiquity. A proposed altered migration of these homologous neurons and circuits during development is offered as a mechanism that may account for the altered configuration of mammalian telencephalae.
Keywords: auditory; birds; microcircuitry; nuclear to laminar transformation; radial columns; reptiles.
© 2015 The Author(s).
Figures
Similar articles
-
Molecular anatomy of the alligator dorsal telencephalon.J Comp Neurol. 2018 Jul 1;526(10):1613-1646. doi: 10.1002/cne.24427. Epub 2018 Apr 17. J Comp Neurol. 2018. PMID: 29520780 Free PMC article.
-
Homology and evolutionary origins of the 'neocortex'.Brain Behav Evol. 1991;38(4-5):264-72. doi: 10.1159/000114393. Brain Behav Evol. 1991. PMID: 1777808 Review.
-
Laminar and columnar auditory cortex in avian brain.Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12676-81. doi: 10.1073/pnas.1006645107. Epub 2010 Jun 28. Proc Natl Acad Sci U S A. 2010. PMID: 20616034 Free PMC article.
-
The evolution of the dorsal pallium in the telencephalon of amniotes: cladistic analysis and a new hypothesis.Brain Res Brain Res Rev. 1994 Jan;19(1):66-101. doi: 10.1016/0165-0173(94)90004-3. Brain Res Brain Res Rev. 1994. PMID: 8167660 Review.
-
Anatomical organization of the visual dorsal ventricular ridge in the chick (Gallus gallus): Layers and columns in the avian pallium.J Comp Neurol. 2015 Dec 1;523(17):2618-36. doi: 10.1002/cne.23808. Epub 2015 Jun 8. J Comp Neurol. 2015. PMID: 25982840
Cited by
-
Active sensing associated with spatial learning reveals memory-based attention in an electric fish.J Neurophysiol. 2016 May 1;115(5):2577-92. doi: 10.1152/jn.00979.2015. Epub 2016 Mar 9. J Neurophysiol. 2016. PMID: 26961107 Free PMC article.
-
Unraveling the Evolutionary Determinants of Sleep.Curr Biol. 2016 Oct 24;26(20):R1073-R1087. doi: 10.1016/j.cub.2016.08.068. Curr Biol. 2016. PMID: 27780049 Free PMC article. Review.
-
Molecular anatomy of the alligator dorsal telencephalon.J Comp Neurol. 2018 Jul 1;526(10):1613-1646. doi: 10.1002/cne.24427. Epub 2018 Apr 17. J Comp Neurol. 2018. PMID: 29520780 Free PMC article.
-
Could theropod dinosaurs have evolved to a human level of intelligence?J Comp Neurol. 2023 Jun;531(9):975-1006. doi: 10.1002/cne.25458. Epub 2023 Apr 7. J Comp Neurol. 2023. PMID: 37029483 Free PMC article. Review.
-
Functional interactions among neurons within single columns of macaque V1.Elife. 2022 Nov 2;11:e79322. doi: 10.7554/eLife.79322. Elife. 2022. PMID: 36321687 Free PMC article.
References
-
- Darwin C. 1859. On the origin of species by means of natural selection. London, UK: John Murray.
-
- Darwin C. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray.
-
- Ariens-Kappers CUA, Huber G, Crosby EC. 1936. The comparative anatomy of the nervous system of vertebrates including man. New York, NY: Macmillan.
-
- Herrick CJ. 1948. The brain of the tiger salamander. Chicago, IL: University of Chicago Press.
-
- Hughlings Jackson J. 1884. Evolution and dissolution of the nervous system. Croonian Lectures delivered at the Royal College of Physicians, March 1884. Lancet 1, 555–558, 649–652, 739–744.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

