Selective capture of glycoproteins using lectin-modified nanoporous gold monolith
- PMID: 26554297
- PMCID: PMC4659647
- DOI: 10.1016/j.chroma.2015.10.060
Selective capture of glycoproteins using lectin-modified nanoporous gold monolith
Abstract
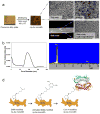
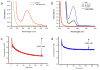
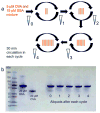
The surface of nanoporous gold (np-Au) monoliths was modified via a flow method with the lectin Concanavalin A (Con A) to develop a substrate for separation and extraction of glycoproteins. Self-assembled monolayers (SAMs) of α-lipoic acid (LA) on the np-Au monoliths were prepared followed by activation of the terminal carboxyl groups to create amine reactive esters that were utilized in the immobilization of Con A. Thermogravimetric analysis (TGA) was used to determine the surface coverages of LA and Con A on np-Au monoliths which were found to be 1.31×10(18) and 1.85×10(15)moleculesm(-2), respectively. An in situ solution depletion method was developed that enabled surface coverage characterization without damaging the substrate and suggesting the possibility of regeneration. Using this method, the surface coverages of LA and Con A were found to be 0.989×10(18) and 1.32×10(15)moleculesm(-2), respectively. The selectivity of the Con A-modified np-Au monolith for the high mannose-containing glycoprotein ovalbumin (OVA) versus negative control non-glycosylated bovine serum albumin (BSA) was demonstrated by the difference in the ratio of the captured molecules to the immobilized Con A molecules, with OVA:Con A=2.3 and BSA:Con A=0.33. Extraction of OVA from a 1:3 mole ratio mixture with BSA was demonstrated by the greater amount of depletion of OVA concentration during the circulation with the developed substrate. A significant amount of captured OVA was eluted using α-methyl mannopyranoside as a competitive ligand. This work is motivated by the need to develop new materials for chromatographic separation and extraction substrates for use in preparative and analytical procedures in glycomics.
Keywords: Chromatography; Glycomics; Glycoprotein; Lectin; Monolith; Nanoporous gold.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures


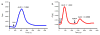
Similar articles
-
Lectin-carbohydrate interactions on nanoporous gold monoliths.New J Chem. 2013 Jul 1;37(7):2150-2165. doi: 10.1039/C3NJ00253E. New J Chem. 2013. PMID: 24883017 Free PMC article.
-
Concanavalin A-immobilized magnetic nanoparticles for selective enrichment of glycoproteins and application to glycoproteomics in hepatocelluar carcinoma cell line.Proteomics. 2010 May;10(10):2000-14. doi: 10.1002/pmic.200900377. Proteomics. 2010. PMID: 20217867
-
Reversible Concanavalin A (Con A) ligands immobilization on metal chelated macroporous cellulose monolith and its selective adsorption for glycoproteins.J Chromatogr A. 2018 May 4;1548:37-43. doi: 10.1016/j.chroma.2018.03.028. Epub 2018 Mar 15. J Chromatogr A. 2018. PMID: 29580801
-
Development and Applications of the Lectin Microarray.Top Curr Chem. 2015;367:105-24. doi: 10.1007/128_2014_612. Top Curr Chem. 2015. PMID: 25821171 Review.
-
Natural and artificial carbohydrate-glued protein aggregates.Adv Biophys. 1997;34:253-62. doi: 10.1016/s0065-227x(97)89643-8. Adv Biophys. 1997. PMID: 9204138 Review.
Cited by
-
Electrochemical impedance spectroscopy study of carbohydrate-terminated alkanethiol monolayers on nanoporous gold: Implications for pore wetting.J Electroanal Chem (Lausanne). 2016 Dec 1;782:174-181. doi: 10.1016/j.jelechem.2016.10.013. Epub 2016 Oct 11. J Electroanal Chem (Lausanne). 2016. PMID: 28413373 Free PMC article.
-
Preparation, Modification, Characterization, and Biosensing Application of Nanoporous Gold Using Electrochemical Techniques.Nanomaterials (Basel). 2018 Mar 16;8(3):171. doi: 10.3390/nano8030171. Nanomaterials (Basel). 2018. PMID: 29547580 Free PMC article. Review.
-
Nanotechnology in Glycomics: Applications in Diagnostics, Therapy, Imaging, and Separation Processes.Med Res Rev. 2017 May;37(3):514-626. doi: 10.1002/med.21420. Epub 2016 Nov 15. Med Res Rev. 2017. PMID: 27859448 Free PMC article. Review.
-
Nanoporous metals by alloy corrosion: Bioanalytical and biomedical applications.MRS Bull. 2018 Jan;43(1):49-56. doi: 10.1557/mrs.2017.298. Epub 2018 Jan 10. MRS Bull. 2018. PMID: 32684663 Free PMC article.
-
Nanoporous Gold Monolith for High Loading of Unmodified Doxorubicin and Sustained Co-Release of Doxorubicin-Rapamycin.Nanomaterials (Basel). 2021 Jan 15;11(1):208. doi: 10.3390/nano11010208. Nanomaterials (Basel). 2021. PMID: 33467416 Free PMC article.
References
-
- Brandley BK, Schnaar RL. Cell-surface carbohydrates in cell recognition and response. J Leukoc Biol. 1986;40:97–111. - PubMed
-
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291:2364–9. - PubMed
-
- Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, Oettgen HF. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978;299:448–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

