Antibody induced by one-dose varicella vaccine soon became weak in children: evidence from a cross-sectional seroepidemiological survey in Beijing, PRC
- PMID: 26554449
- PMCID: PMC4641405
- DOI: 10.1186/s12879-015-1236-x
Antibody induced by one-dose varicella vaccine soon became weak in children: evidence from a cross-sectional seroepidemiological survey in Beijing, PRC
Abstract
Background: Numerous post-licensure studies, mostly from field epidemiological evidences such as outbreak surveys, have demonstrated the effectivenesss and insufficiency of one-dose varicella vaccine in outbreak control. Serological evidence of immunization failure is, however, relatively less reported in contrast. A cross-sectional seroepidemiological survey of Beijing residents was performed in 2012 in the People's Republic of China, after the one-dose varicella vaccine had been widely used for several years.
Methods: Multistage stratified random sampling method was designed to recruit 2 144 subjects. The ELISA method was used to test the present blood samples collected and the reserve samples collected in 2008 to assess the trends of anti-VZV seroprevalence in the past 5 years and to determine the risk factors for varicella infection.
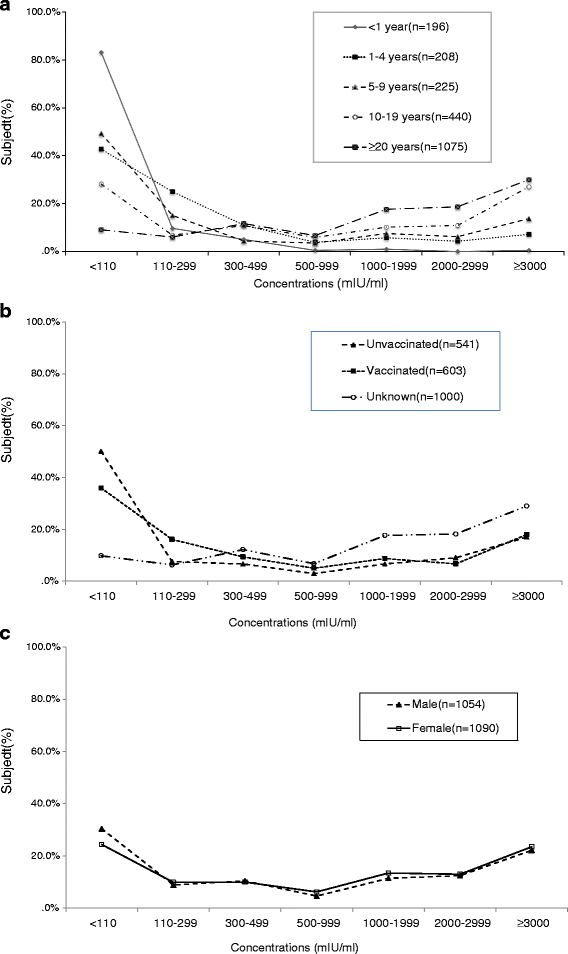
Results: The age- and sex- adjusted overall anti-VZV seropositivity of Beijing residents in 2012 was 84.5%. Two groups' adjusted overall anti-VZV seroprevalence in 2012 showed obvious growth compared with 2008 (<1 yr old: from 6.3% to 16.9%; 1-4 yr old: from 27.6% to 57.2%). Reported one-dose vaccination history was 71.6% (149/208), 80.9% (182/225) and 82.2% (180/219) in the 1-4 yr, 5-9 yr, 10-14 yr age groups, respectively. Of subjects who had received the one-dose vaccine, 36% (216/603) showed negative anti-VZV concentrations (<110 mIU/mL); additionally 15.9% (96/603) of such subjects' anti-VZV concentrations were in the lowest positive concentration group (110-299 mIU/mL). Seropositivity in permanent residents of 1-9 yr old with verified vaccination was merely 61.8%. Various age groups (1-3 yr, 4-6 yr, and 7-9 yr) all showed seropositivity that gradually decreased with increasing of the interval between vaccination and blood sampling.
Conclusion: Mass varicella vaccination significantly improved the immunity of younger Beijing residents. However, vaccine-induced anti-VZV antibody soon became weak in children with high coverage (approximately 80%) after vaccination for several years which is significantly higher than reported in pre-licensure studies. A government-funded 2-dose immunization program with mandatory vaccination schedule for Beijing residents may need consideration in the near future.
Figures
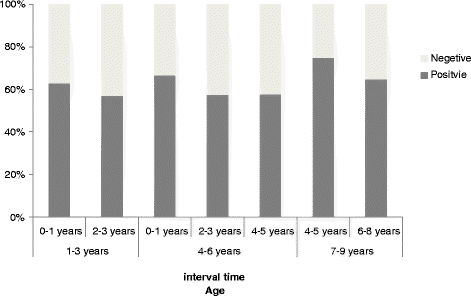
Similar articles
-
Varicella-zoster virus seroprevalence in children and adolescents in the pre-varicella vaccine era, Germany.BMC Infect Dis. 2017 May 19;17(1):356. doi: 10.1186/s12879-017-2461-2. BMC Infect Dis. 2017. PMID: 28525973 Free PMC article.
-
Seroepidemiology of varicella in Hangzhou, China in the vaccine era.Hum Vaccin Immunother. 2018;14(10):2464-2471. doi: 10.1080/21645515.2018.1477909. Epub 2018 Jul 30. Hum Vaccin Immunother. 2018. PMID: 30019992 Free PMC article.
-
Hospital-based seroepidemiological analysis of varicella antibodies in children without history of varicella disease.J Infect Dev Ctries. 2025 Mar 31;19(3):452-457. doi: 10.3855/jidc.20424. J Infect Dev Ctries. 2025. PMID: 40168390
-
Long-term clinical studies of varicella vaccine at a regional hospital in Japan and proposal for a varicella vaccination program.Vaccine. 2013 Dec 9;31(51):6155-60. doi: 10.1016/j.vaccine.2013.10.060. Epub 2013 Oct 30. Vaccine. 2013. PMID: 24183712 Review.
-
Varicella vaccination in Japan: necessity of implementing a routine vaccination program.J Infect Chemother. 2013 Apr;19(2):188-95. doi: 10.1007/s10156-013-0577-x. Epub 2013 Mar 13. J Infect Chemother. 2013. PMID: 23483311 Review.
Cited by
-
Analysis of sero-epidemiological characteristics of varicella in healthy children in Jiangsu Province, China.BMC Infect Dis. 2018 Nov 14;18(1):563. doi: 10.1186/s12879-018-3496-8. BMC Infect Dis. 2018. PMID: 30428851 Free PMC article.
-
A persistent outbreak of varicella in a primary school in Dongguan City, Guangdong Province, China.J Int Med Res. 2020 Mar;48(3):300060519887847. doi: 10.1177/0300060519887847. Epub 2019 Nov 27. J Int Med Res. 2020. PMID: 31771379 Free PMC article.
-
Impact of varicella vaccination in Argentina: Seroprevalence in children and adults in a pediatric hospital.Vaccine X. 2021 Dec 21;10:100136. doi: 10.1016/j.jvacx.2021.100136. eCollection 2022 Apr. Vaccine X. 2021. PMID: 35024601 Free PMC article.
-
The impact of long-term moderate level of vaccination coverage for epidemiology of varicella in Lu'an, China: should we change immunisation strategy now?Epidemiol Infect. 2020 Mar 13;148:e74. doi: 10.1017/S0950268820000667. Epidemiol Infect. 2020. PMID: 32167037 Free PMC article.
-
Seroprevalence of varicella-zoster virus as measured by fluorescent antibody to membrane antigen assay and glycoprotein enzyme-linked immunosorbent assay more than 10 years after initiation of a universal vaccination program: An observational study.Medicine (Baltimore). 2024 Jan 19;103(3):e36931. doi: 10.1097/MD.0000000000036931. Medicine (Baltimore). 2024. PMID: 38241578 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

