Production of RANKL by Memory B Cells: A Link Between B Cells and Bone Erosion in Rheumatoid Arthritis
- PMID: 26554541
- PMCID: PMC4956406
- DOI: 10.1002/art.39489
Production of RANKL by Memory B Cells: A Link Between B Cells and Bone Erosion in Rheumatoid Arthritis
Abstract
Objective: Rheumatoid arthritis (RA) is a systemic autoimmune disease that often leads to joint damage. The mechanisms of bone damage in RA are complex, involving activation of bone-resorbing osteoclasts (OCs) by synoviocytes and Th17 cells. This study was undertaken to investigate whether B cells play a direct role in osteoclastogenesis through the production of RANKL, the essential cytokine for OC development.
Methods: RANKL production by total B cells or sorted B cell subpopulations in the peripheral blood and synovial tissue from healthy donors or anti-cyclic citrullinated peptide-positive patients with RA was examined by flow cytometry, real-time polymerase chain reaction, enzyme-linked immunosorbent assay, and immunohistochemical analysis. To define direct effects on osteoclastogenesis, B cells were cocultured with CD14+ monocytes, and OCs were enumerated by tartrate-resistant acid phosphatase staining.
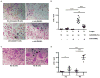
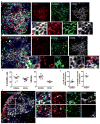
Results: Healthy donor peripheral blood B cells were capable of expressing RANKL upon stimulation, with switched memory B cells (CD27+IgD-) having the highest propensity for RANKL production. Notably, switched memory B cells in the peripheral blood from RA patients expressed significantly more RANKL compared to healthy controls. In RA synovial fluid and tissue, memory B cells were enriched and spontaneously expressed RANKL, with some of these cells visualized adjacent to RANK+ OC precursors. Critically, B cells supported OC differentiation in vitro in a RANKL-dependent manner, and the number of OCs was higher in cultures with RA B cells than in those derived from healthy controls.
Conclusion: These findings reveal the critical importance of B cells in bone homeostasis and their likely contribution to joint destruction in RA.
© 2016, American College of Rheumatology.
Figures



References
-
- Scott DL, Pugner K, Kaarela K, Doyle DV, Woolf A, Holmes J, et al. The links between joint damage and disability in rheumatoid arthritis. Rheumatology (Oxford) 2000;39:122–32. - PubMed
-
- Hirayama T, Danks L, Sabokbar A, Athanasou NA. Osteoclast formation and activity in the pathogenesis of osteoporosis in rheumatoid arthritis. Rheumatology (Oxford) 2002;41:1232–9. - PubMed
-
- Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

