The Cardiovascular Trial of the Testosterone Trials: rationale, design, and baseline data of a clinical trial using computed tomographic imaging to assess the progression of coronary atherosclerosis
- PMID: 26554661
- PMCID: PMC4738150
- DOI: 10.1097/MCA.0000000000000321
The Cardiovascular Trial of the Testosterone Trials: rationale, design, and baseline data of a clinical trial using computed tomographic imaging to assess the progression of coronary atherosclerosis
Abstract
Background: Data from prior studies have yielded inconsistent results on the association of serum testosterone levels with the risk for cardiovascular disease. There are no clinical trial data on the effects of testosterone replacement therapy on plaque progression.
Objective: We designed a study to investigate the effect of testosterone therapy on coronary artery plaque progression using serial coronary computed tomographic angiography (CCTA). In this paper, we describe the study design, methods, and characteristics of the study population.
Methods: The Cardiovascular Trial of the Testosterone Trials (TTrials; NCT00799617) is a double-blind, placebo-controlled trial of 1 year of testosterone therapy in men 65 years or older with clinical manifestations of androgen deficiency and unequivocally low serum testosterone concentrations (<275 ng/dl). CCTA performed at baseline and after 12 months of therapy will determine the effects of testosterone on the progression of the total volume of noncalcified plaques. All scans are evaluated at a central reading center by an investigator blinded to treatment assignment.
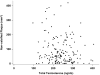
Results: A total of 165 men were enrolled. The average age is 71.1 years, and the average BMI is 30.7. About 9% of men had a history of myocardial infarction, 6% angina, and 10% coronary artery revascularization. A majority reported hypertension and/or high cholesterol; 31.8% reported diabetes. Total noncalcified plaque at baseline showed a slight but nonsignificant trend toward lower plaque volume with higher serum testosterone concentrations (P=0.12).
Conclusion: The Cardiovascular Trial will test the hypothesis that testosterone therapy inhibits coronary plaque progression, as assessed by serial CCTA.
Conflict of interest statement
Figures

References
-
- Vermeulen A, Rubens R, Verdonck L. Testosterone secretion and metabolism in male senescene. J Clin Endocrinol Metab. 1972;34:730–735. - PubMed
-
- Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87(2):589–598. - PubMed
-
- Harman S, Metter E, Tobin J, Pearson J, Blackman M. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. - PubMed
-
- Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78(3):539–545. - PubMed
-
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25(1):55–60. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- DK-079626/DK/NIDDK NIH HHS/United States
- U01 AG030644/AG/NIA NIH HHS/United States
- R01 AG037679/AG/NIA NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- R01 DK031801/DK/NIDDK NIH HHS/United States
- R37 AG007181/AG/NIA NIH HHS/United States
- R01 AG028507/AG/NIA NIH HHS/United States
- AG028507/AG/NIA NIH HHS/United States
- 5-U01-AG030644/AG/NIA NIH HHS/United States
- T32 DK007571/DK/NIDDK NIH HHS/United States
- R01 AG007181/AG/NIA NIH HHS/United States
- AG07181/AG/NIA NIH HHS/United States
- T32-DK007571/DK/NIDDK NIH HHS/United States
- P60 DK079626/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

