Langerhans cell origin and regulation
- PMID: 26554892
- PMCID: PMC4685746
- DOI: 10.1097/MOH.0000000000000202
Langerhans cell origin and regulation
Abstract
Purpose of review: This article summarizes recent research on the ontogeny of Langerhans cells and regulation of their homeostasis in quiescent and inflamed conditions.
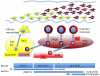
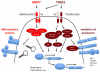
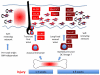
Recent findings: Langerhans cells originate prenatally and may endure throughout life, independently of bone marrow-derived precursors. Fate-mapping experiments have recently resolved the relative contribution of primitive yolk sac and fetal liver hematopoiesis to the initial formation of Langerhans cells. In postnatal life, local self-renewal restores Langerhans cell numbers following chronic or low-grade inflammatory insults. However, severe inflammation recruits de-novo bone marrow-derived precursors in two waves; a transient population of classical monocytes followed by uncharacterized myeloid precursors that form a stable self-renewing Langerhans cell network as inflammation subsides. Human CD1c⁺ dendritic cells have Langerhans cell potential in vitro, raising the possibility that dendritic cell progenitors provide the second wave. Langerhans cell development depends upon transforming growth factor beta receptor signaling with distinct pathways active during differentiation and homeostasis. Langerhans cell survival is mediated by multiple pathways including mechanistic target of rapamycin and extracellular signal-regulated kinase signaling, mechanisms that become highly relevant in Langerhans cell neoplasia.
Summary: The study of Langerhans cells continues to provide novel and unexpected insights into the origin and regulation of myeloid cell populations. The melding of macrophage and dendritic cell biology, shaped by a unique habitat, is a special feature of Langerhans cells.
Figures
References
-
- Romani N, Brunner PM, Stingl G. Changing views of the role of Langerhans cells. J Invest Dermatol. 2012;132:872–881. - PubMed
-
- Langerhans P. Uber die Nerven der menschlichen Haut. VirchowsArchpathol. 1868;44:325–337.
-
- Stingl G, Wolff-Schreiner EC, Pichler WJ, Gschnait F, Knapp W, Wolff K. Epidermal Langerhans cells bear Fc and C3 receptors. Nature. 1977;268:245–246. - PubMed
-
- Klareskog L, Tjernlund U, Forsum U, Peterson PA. Epidermal Langerhans cells express Ia antigens. Nature. 1977;268:248–250. - PubMed
-
- Katz SI, Tamaki K, Sachs DH. Epidermal Langerhans cells are derived from cells originating in bone marrow. Nature. 1979;282:324–36. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

