Neurological Response to cART vs. cART plus Integrase Inhibitor and CCR5 Antagonist Initiated during Acute HIV
- PMID: 26555069
- PMCID: PMC4640512
- DOI: 10.1371/journal.pone.0142600
Neurological Response to cART vs. cART plus Integrase Inhibitor and CCR5 Antagonist Initiated during Acute HIV
Abstract
Objective: To compare central nervous system (CNS) outcomes in participants treated during acute HIV infection with standard combination antiretroviral therapy (cART) vs. cART plus integrase inhibitor and CCR5 antagonist (cART+).
Design: 24-week randomized open-label prospective evaluation.
Method: Participants were evaluated then randomized to initiate cART (efavirenz, tenofovir, and either emtricitabine or lamivudine) vs. cART+ (cART plus raltegravir and maraviroc) during acute HIV and re-evaluated at 4, 12 and 24 weeks. We examined plasma and CSF cytokines, HIV RNA levels, neurological and neuropsychological findings, and brain MRS across groups and compared to healthy controls.
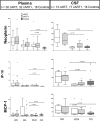
Results: At baseline, 62 participants were in Fiebig stages I-V. Randomized groups were similar for mean age (27 vs. 25, p = 0.137), gender (each 94% male), plasma log10 HIV RNA (5.4 vs. 5.6, p = 0.382), CSF log10 HIV RNA (2.35 vs. 3.31, p = 0.561), and estimated duration of HIV (18 vs. 17 days, p = 0.546). Randomized arms did not differ at 24 weeks by any CNS outcome. Combining arms, all measures concurrent with antiretroviral treatment improved, for example, neuropsychological testing (mean NPZ-4 of -0.408 vs. 0.245, p<0.001) and inflammatory markers by MRS (e.g. mean frontal white matter (FWM) choline of 2.92 vs. 2.84, p = 0.045) at baseline and week 24, respectively. Plasma neopterin (p<0.001) and interferon gamma-induced protein 10 (IP-10) (p = 0.007) remained elevated in participants compared to controls but no statistically significant differences were seen in CSF cytokines compared to controls, despite individual variability among the HIV-infected group.
Conclusions: A 24-week course of cART+ improved CNS related outcomes, but was not associated with measurable differences compared to standard cART.
Conflict of interest statement
Figures

References
-
- Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206(2):275–82 (PMID:22551810; PMCID:3490695). Epub 2012/05/04. doi: jis326 [pii] 10.1093/infdis/jis326 - DOI - PMC - PubMed
-
- Spudich S, Gisslen M, Hagberg L, Lee E, Liegler T, Brew B, et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J Infect Dis. 2011;204(5):753–60. Epub 2011/08/17. 10.1093/infdis/jir387 ; PubMed Central PMCID: PMCPmc3156103. - DOI - PMC - PubMed
-
- Peluso MJ, Meyerhoff DJ, Price RW, Peterson J, Lee E, Young AC, et al. Cerebrospinal Fluid and Neuroimaging Biomarker Abnormalities Suggest Early Neurological Injury in a Subset of Individuals During Primary HIV Infection. J Infect Dis. 2013;207(11):1703–12. Epub 2013/03/06. 10.1093/infdis/jit088 - DOI - PMC - PubMed
-
- Peluso M, Valcour V, Ananworanich J, Sithinamsuwan P, Chalermchai T, Chomchey N, et al. Absence of cerebrospinal fluid signs of neuronal injury before and after immediate antiretroviral therapy in acute HIV infection. Conference on Retrovirus and Opportunistic Infections (CROI); 2014; Seattle, WA. - PMC - PubMed
-
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

