Selective Inhibition of Bacterial Tryptophanyl-tRNA Synthetases by Indolmycin Is Mechanism-based
- PMID: 26555258
- PMCID: PMC4697160
- DOI: 10.1074/jbc.M115.690321
Selective Inhibition of Bacterial Tryptophanyl-tRNA Synthetases by Indolmycin Is Mechanism-based
Abstract
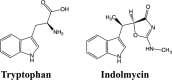
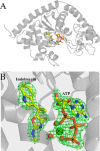
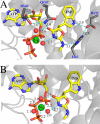
Indolmycin is a natural tryptophan analog that competes with tryptophan for binding to tryptophanyl-tRNA synthetase (TrpRS) enzymes. Bacterial and eukaryotic cytosolic TrpRSs have comparable affinities for tryptophan (Km ∼ 2 μm), and yet only bacterial TrpRSs are inhibited by indolmycin. Despite the similarity between these ligands, Bacillus stearothermophilus (Bs)TrpRS preferentially binds indolmycin ∼1500-fold more tightly than its tryptophan substrate. Kinetic characterization and crystallographic analysis of BsTrpRS allowed us to probe novel aspects of indolmycin inhibitory action. Previous work had revealed that long range coupling to residues within an allosteric region called the D1 switch of BsTrpRS positions the Mg(2+) ion in a manner that allows it to assist in transition state stabilization. The Mg(2+) ion in the inhibited complex forms significantly closer contacts with non-bridging oxygen atoms from each phosphate group of ATP and three water molecules than occur in the (presumably catalytically competent) pre-transition state (preTS) crystal structures. We propose that this altered coordination stabilizes a ground state Mg(2+)·ATP configuration, accounting for the high affinity inhibition of BsTrpRS by indolmycin. Conversely, both the ATP configuration and Mg(2+) coordination in the human cytosolic (Hc)TrpRS preTS structure differ greatly from the BsTrpRS preTS structure. The effect of these differences is that catalysis occurs via a different transition state stabilization mechanism in HcTrpRS with a yet-to-be determined role for Mg(2+). Modeling indolmycin into the tryptophan binding site points to steric hindrance and an inability to retain the interactions used for tryptophan substrate recognition as causes for the 1000-fold weaker indolmycin affinity to HcTrpRS.
Keywords: aminoacyl-tRNA synthetase; enzyme catalysis; enzyme inhibitor; inhibition; inhibition by ground-state stabilization; inhibition mechanism; thermofluor; x-ray crystallography.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Werner R. G., Thorpe L. F., Reuter W., and Nierhaus K. H. (1976) Indolmycin inhibits prokaryotic tryptophanyl-tRNA ligase. Eur. J. Biochem. 68, 1–3 - PubMed
-
- Nakama T., Nureki O., and Yokoyama S. (2001) Structural basis for the recognition of isoleucyl-adenylate and an antibiotic, mupirocin, by isoleucyl-tRNA synthetase. J. Biol. Chem. 276, 47387–47393 - PubMed
-
- Konrad I., and R. (1977) Inhibition of phenylalanine tRNA synthetase from Bacillus subtilis by ochratoxin A. FEBS Lett. 83, 341–347 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

