Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants
- PMID: 26555265
- PMCID: PMC4714196
- DOI: 10.1074/jbc.M115.683011
Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants
Abstract
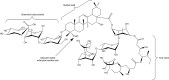
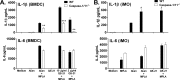
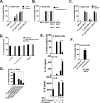
Many immunostimulants act as vaccine adjuvants via activation of the innate immune system, although in many cases it is unclear which specific molecules contribute to the stimulatory activity. QS-21 is a defined, highly purified, and soluble saponin adjuvant currently used in licensed and exploratory vaccines, including vaccines against malaria, cancer, and HIV-1. However, little is known about the mechanisms of cellular activation induced by QS-21. We observed QS-21 to elicit caspase-1-dependent IL-1β and IL-18 release in antigen-presenting cells such as macrophages and dendritic cells when co-stimulated with the TLR4-agonist adjuvant monophosphoryl lipid A. Furthermore, our data suggest that the ASC-NLRP3 inflammasome is responsible for QS-21-induced IL-1β/IL-18 release. At higher concentrations, QS-21 induced macrophage and dendritic cell death in a caspase-1-, ASC-, and NLRP3-independent manner, whereas the presence of cholesterol rescued cell viability. A nanoparticulate adjuvant that contains QS-21 as part of a heterogeneous mixture of saponins also induced IL-1β in an NLRP3-dependent manner. Interestingly, despite the role NLRP3 plays for cellular activation in vitro, NLRP3-deficient mice immunized with HIV-1 gp120 and QS-21 showed significantly higher levels of Th1 and Th2 antigen-specific T cell responses and increased IgG1 and IgG2c compared with wild type controls. Thus, we have identified QS-21 as a nonparticulate single molecular saponin that activates the NLRP3 inflammasome, but this signaling pathway may contribute to decreased antigen-specific responses in vivo.
Keywords: NLRP3; Toll-like receptor 4 (TLR4); adjuvants*; caspase 1 (CASP1); human immunodeficiency virus (HIV); inflammasome; monophosphoryl lipid A; saponin; vaccine.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




Similar articles
-
Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review.Phytomedicine. 2019 Jul;60:152905. doi: 10.1016/j.phymed.2019.152905. Epub 2019 Mar 30. Phytomedicine. 2019. PMID: 31182297 Free PMC article. Review.
-
Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant.J Immunol. 2014 Apr 1;192(7):3259-68. doi: 10.4049/jimmunol.1302011. Epub 2014 Mar 7. J Immunol. 2014. PMID: 24610009
-
Advanced glycation end products impair NLRP3 inflammasome-mediated innate immune responses in macrophages.J Biol Chem. 2017 Dec 15;292(50):20437-20448. doi: 10.1074/jbc.M117.806307. Epub 2017 Oct 19. J Biol Chem. 2017. PMID: 29051224 Free PMC article.
-
The adjuvant combination monophosphoryl lipid A and QS21 switches T cell responses induced with a soluble recombinant HIV protein from Th2 to Th1.Vaccine. 1999 Jun 4;17(20-21):2517-27. doi: 10.1016/s0264-410x(99)00062-6. Vaccine. 1999. PMID: 10418898
-
Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants.Trends Pharmacol Sci. 2018 Jun;39(6):573-585. doi: 10.1016/j.tips.2018.03.005. Epub 2018 Apr 11. Trends Pharmacol Sci. 2018. PMID: 29655658 Review.
Cited by
-
Interferon-induced guanylate-binding proteins in inflammasome activation and host defense.Nat Immunol. 2016 May;17(5):481-9. doi: 10.1038/ni.3440. Nat Immunol. 2016. PMID: 27092805 Free PMC article.
-
Plasmodium falciparum Malaria Vaccines and Vaccine Adjuvants.Vaccines (Basel). 2021 Sep 24;9(10):1072. doi: 10.3390/vaccines9101072. Vaccines (Basel). 2021. PMID: 34696180 Free PMC article. Review.
-
Vaccine Strategies to Improve Anti-cancer Cellular Immune Responses.Front Immunol. 2019 Jan 22;10:8. doi: 10.3389/fimmu.2019.00008. eCollection 2019. Front Immunol. 2019. PMID: 30723469 Free PMC article. Review.
-
Adjuvants: Engineering Protective Immune Responses in Human and Veterinary Vaccines.Methods Mol Biol. 2022;2412:179-231. doi: 10.1007/978-1-0716-1892-9_9. Methods Mol Biol. 2022. PMID: 34918246 Review.
-
Central Role of CD169+ Lymph Node Resident Macrophages in the Adjuvanticity of the QS-21 Component of AS01.Sci Rep. 2016 Dec 20;6:39475. doi: 10.1038/srep39475. Sci Rep. 2016. PMID: 27996000 Free PMC article.
References
-
- Chen M., Wang H., Chen W., and Meng G. (2011) Regulation of adaptive immunity by the NLRP3 inflammasome. Int. Immunopharmacol. 11, 549–554 - PubMed
-
- Tschopp J., and Schroder K. (2010) NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 AI117706/AI/NIAID NIH HHS/United States
- AI117706/AI/NIAID NIH HHS/United States
- T32 AI095213/AI/NIAID NIH HHS/United States
- AI095213/AI/NIAID NIH HHS/United States
- AI057588/AI/NIAID NIH HHS/United States
- AI07538/AI/NIAID NIH HHS/United States
- U19 AI082676/AI/NIAID NIH HHS/United States
- R01 AI108834/AI/NIAID NIH HHS/United States
- T32 AI007538/AI/NIAID NIH HHS/United States
- 5 P01 AI082274/AI/NIAID NIH HHS/United States
- P01 AI082274/AI/NIAID NIH HHS/United States
- R01 AI057588/AI/NIAID NIH HHS/United States
- 5 U19 AI082676/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

