Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor α agents, a retrospective cohort study
- PMID: 26555431
- PMCID: PMC4641344
- DOI: 10.1186/s13075-015-0835-7
Incidence and complications of interstitial lung disease in users of tocilizumab, rituximab, abatacept and anti-tumor necrosis factor α agents, a retrospective cohort study
Abstract
Introduction: Interstitial lung disease (ILD) is a common extra-articular condition in rheumatoid arthritis (RA), but few studies have systematically investigated its incidence and risk factors in patients receiving anti-tumor necrosis factor-alpha (anti-TNFα) agents or alternate mechanisms of action (MOAs) (e.g., T-cell, B-cell, and interleukin-6 inhibitors).
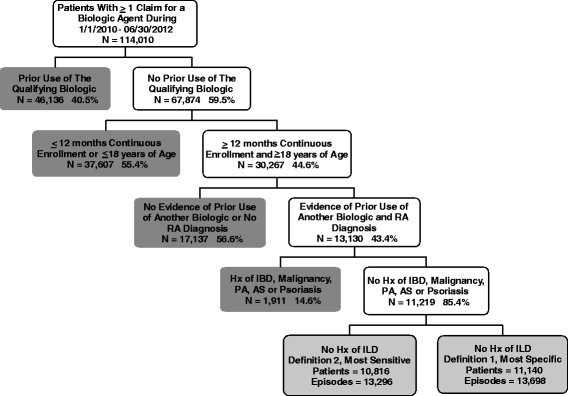
Methods: RA patients at least 18 years old were selected from the MarketScan databases (2010-2012) if they had at least one prescription/administration of abatacept, rituximab, tocilizumab, or anti-TNF after having discontinued a different biologic agent and meeting enrollment criteria. Cox models estimated the risk of incident ILD and ILD-related hospitalization. Sensitivity analyses used an alternate ILD case definition.
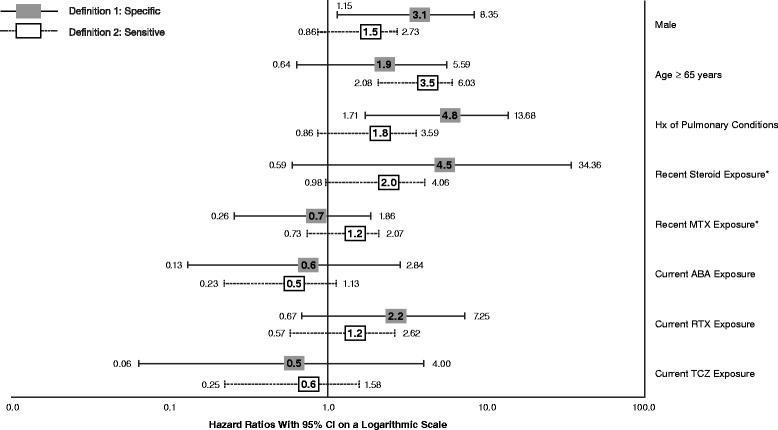
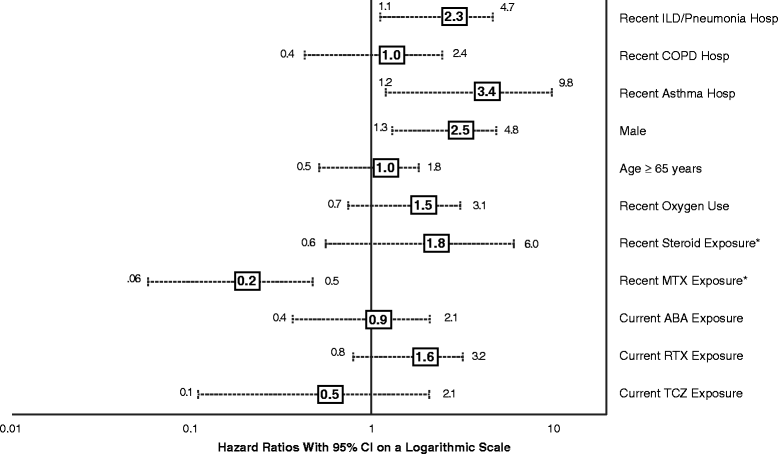
Results: We identified 13,795 episodes of biologic exposure in 11,219 patients. Mean (standard deviation) follow-up was 0.7 (0.5) years. Patients receiving alternate MOA agents were more likely to have had recent exposure to steroids, prior exposure to a greater number of biologics, and history of ILD, anemia, chronic obstructive pulmonary disease, and other pulmonary conditions. When the sensitive definition was used, unadjusted ILD incidence rates (95% confidence interval, or CI) ranged from 4.0 (1.6-8.2, abatacept) to 12.2 (5.6-23.2, infliximab) per 1000 person-years. Being older (hazard ratio (HR) 3.5; 95% CI 2.1-6.0), being male (HR 3.1; 95% CI 1.2-8.4), and having another pulmonary condition (HR 4.8; 95% CI 1.7-13.7) were associated with increased ILD incidence in either sensitive and/or specific models. There were no significant differences by biologic class. Hospitalization rates (95% CI) when the sensitive definition was used ranged from 55.6 (6.7-200.7, tocilizumab) to 262.5 (71.5-672.2, infliximab). In Cox models, recent methotrexate exposure was associated with reduced ILD hospitalization (HR 0.16; 95% CI 0.06-0.46), whereas being male (HR 2.5; 95% CI 1.3-4.8) and having had a hospitalization for asthma (HR 3.4; 95% CI 1.2-9.8) or ILD/pneumonia (HR 2.3; 95% CI 1.1-4.7) in the 12 months prior to index were associated with increased hospitalization risk.
Conclusions: There were no significant differences in the risk of ILD and its related complications between RA patients receiving anti-TNFα agents and those receiving alternate MOA agents. Further studies are needed that account for differences in baseline characteristics in order to fully evaluate the risk of ILD and its complications.
Figures
Similar articles
-
Incidence of Interstitial Lung Disease in Patients With Rheumatoid Arthritis Treated With Biologic and Targeted Synthetic Disease-Modifying Antirheumatic Drugs.JAMA Netw Open. 2023 Mar 1;6(3):e233640. doi: 10.1001/jamanetworkopen.2023.3640. JAMA Netw Open. 2023. PMID: 36939701 Free PMC article.
-
Comparative Risk of Hospitalized Infection Associated With Biologic Agents in Rheumatoid Arthritis Patients Enrolled in Medicare.Arthritis Rheumatol. 2016 Jan;68(1):56-66. doi: 10.1002/art.39399. Arthritis Rheumatol. 2016. PMID: 26315675
-
Malignant Neoplasms in Patients With Rheumatoid Arthritis Treated With Tumor Necrosis Factor Inhibitors, Tocilizumab, Abatacept, or Rituximab in Clinical Practice: A Nationwide Cohort Study From Sweden.JAMA Intern Med. 2017 Nov 1;177(11):1605-1612. doi: 10.1001/jamainternmed.2017.4332. JAMA Intern Med. 2017. PMID: 28975211 Free PMC article.
-
Lung involvement and drug-induced lung disease in patients with rheumatoid arthritis.Expert Rev Clin Immunol. 2013 Jul;9(7):649-57. doi: 10.1586/1744666X.2013.811173. Expert Rev Clin Immunol. 2013. PMID: 23899235 Review.
-
Therapeutic management of patients with rheumatoid arthritis and associated interstitial lung disease: case report and literature review.Ther Adv Respir Dis. 2017 Jan;11(1):64-72. doi: 10.1177/1753465816668780. Epub 2016 Oct 12. Ther Adv Respir Dis. 2017. PMID: 27733490 Free PMC article. Review.
Cited by
-
Validation of an algorithm to identify incident interstitial lung disease in patients with rheumatoid arthritis.Arthritis Res Ther. 2022 Jan 3;24(1):2. doi: 10.1186/s13075-021-02655-z. Arthritis Res Ther. 2022. PMID: 34980225 Free PMC article.
-
Update on tocilizumab in rheumatoid arthritis: a narrative review.Front Immunol. 2025 Feb 24;16:1470488. doi: 10.3389/fimmu.2025.1470488. eCollection 2025. Front Immunol. 2025. PMID: 40066438 Free PMC article. Review.
-
Incidence of Interstitial Lung Disease in Patients With Rheumatoid Arthritis Treated With Biologic and Targeted Synthetic Disease-Modifying Antirheumatic Drugs.JAMA Netw Open. 2023 Mar 1;6(3):e233640. doi: 10.1001/jamanetworkopen.2023.3640. JAMA Netw Open. 2023. PMID: 36939701 Free PMC article.
-
Management issues in rheumatoid arthritis-associated interstitial lung disease.Curr Opin Rheumatol. 2020 May;32(3):255-263. doi: 10.1097/BOR.0000000000000703. Curr Opin Rheumatol. 2020. PMID: 32141954 Free PMC article. Review.
-
Interstitial Lung Disease in Connective Tissue Disease: A Common Lesion With Heterogeneous Mechanisms and Treatment Considerations.Front Immunol. 2021 Jun 7;12:684699. doi: 10.3389/fimmu.2021.684699. eCollection 2021. Front Immunol. 2021. PMID: 34163483 Free PMC article. Review.
References
-
- Nyhall-Wahlin BM, Petersson IF, Jacobsson C, Geborek P, Nilsson JÅ, Nilsson K, et al. Extra-articular manifestations in a community-based sample of patients with rheumatoid arthritis: incidence and relationship to treatment with TNF inhibitors. Scand J Rheumatol. 2012;41:434–7. doi: 10.3109/03009742.2012.695803. - DOI - PubMed
-
- Olsen AL, Schwarz MI, Roman J. Interstitial Lung Disease. In: Schraufnagel DE, editor. Breathing in America: Diseases, Progress, and Hope. American Thoracic Society. 2010. pp. 99–108.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

