Nitrogen Mustard-Induced Corneal Injury Involves DNA Damage and Pathways Related to Inflammation, Epithelial-Stromal Separation, and Neovascularization
- PMID: 26555588
- PMCID: PMC4706783
- DOI: 10.1097/ICO.0000000000000685
Nitrogen Mustard-Induced Corneal Injury Involves DNA Damage and Pathways Related to Inflammation, Epithelial-Stromal Separation, and Neovascularization
Abstract
Purpose: To evaluate the toxic effects and associated mechanisms in corneal tissue exposed to the vesicating agent, nitrogen mustard (NM), a bifunctional alkylating analog of the chemical warfare agent sulfur mustard.
Methods: Toxic effects and associated mechanisms were examined in maximally affected corneal tissue using corneal cultures and human corneal epithelial (HCE) cells exposed to NM.
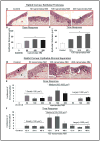
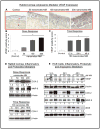
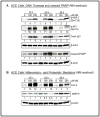
Results: Analysis of ex vivo rabbit corneas showed that NM exposure increased apoptotic cell death, epithelial thickness, epithelial-stromal separation, and levels of vascular endothelial growth factor, cyclooxygenase 2, and matrix metalloproteinase-9. In HCE cells, NM exposure resulted in a dose-dependent decrease in cell viability and proliferation, which was associated with DNA damage in terms of an increase in p53 ser15, total p53, and H2A.X ser139 levels. NM exposure also induced caspase-3 and poly ADP ribose polymerase cleavage, suggesting their involvement in NM-induced apoptotic death in the rabbit cornea and HCE cells. Similar to rabbit cornea, NM exposure caused an increase in cyclooxygenase 2, matrix metalloproteinase-9, and vascular endothelial growth factor levels in HCE cells, indicating a role of these molecules and related pathways in NM-induced corneal inflammation, epithelial-stromal separation, and neovascularization. NM exposure also induced activation of activator protein 1 transcription factor proteins and upstream signaling pathways including mitogen-activated protein kinases and Akt protein kinase, suggesting that these could be key factors involved in NM-induced corneal injury.
Conclusions: Results from this study provide insight into the molecular targets and pathways that could be involved in NM-induced corneal injuries laying the background for further investigation of these pathways in vesicant-induced ocular injuries, which could be helpful in the development of targeted therapies.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose.
Figures



Similar articles
-
Acute corneal injury in rabbits following nitrogen mustard ocular exposure.Exp Mol Pathol. 2019 Oct;110:104275. doi: 10.1016/j.yexmp.2019.104275. Epub 2019 Jun 21. Exp Mol Pathol. 2019. PMID: 31233733 Free PMC article.
-
Histopathological and Molecular Changes in the Rabbit Cornea From Arsenical Vesicant Lewisite Exposure.Toxicol Sci. 2017 Dec 1;160(2):420-428. doi: 10.1093/toxsci/kfx198. Toxicol Sci. 2017. PMID: 28973427 Free PMC article.
-
Nitrogen Mustard-Induced Ex Vivo Human Cornea Injury Model and Therapeutic Intervention by Dexamethasone.J Pharmacol Exp Ther. 2024 Jan 17;388(2):484-494. doi: 10.1124/jpet.123.001760. J Pharmacol Exp Ther. 2024. PMID: 37474260 Free PMC article.
-
Research models of sulfur mustard- and nitrogen mustard-induced ocular injuries and potential therapeutics.Exp Eye Res. 2022 Oct;223:109209. doi: 10.1016/j.exer.2022.109209. Epub 2022 Aug 9. Exp Eye Res. 2022. PMID: 35961426 Review.
-
Corneal toxicity induced by vesicating agents and effective treatment options.Ann N Y Acad Sci. 2016 Jun;1374(1):193-201. doi: 10.1111/nyas.13121. Epub 2016 Jun 21. Ann N Y Acad Sci. 2016. PMID: 27327041 Free PMC article. Review.
Cited by
-
An Engineered Human Fibroblast Growth Factor-1 Derivative, TTHX1114, Ameliorates Short-term Corneal Nitrogen Mustard Injury in Rabbit Organ Cultures.Invest Ophthalmol Vis Sci. 2018 Sep 4;59(11):4720-4730. doi: 10.1167/iovs.18-24568. Invest Ophthalmol Vis Sci. 2018. PMID: 30267094 Free PMC article.
-
Acute corneal injury in rabbits following nitrogen mustard ocular exposure.Exp Mol Pathol. 2019 Oct;110:104275. doi: 10.1016/j.yexmp.2019.104275. Epub 2019 Jun 21. Exp Mol Pathol. 2019. PMID: 31233733 Free PMC article.
-
A Practical and Safe Model of Nitrogen Mustard Injury in Cornea.bioRxiv [Preprint]. 2024 Oct 22:2024.10.18.619116. doi: 10.1101/2024.10.18.619116. bioRxiv. 2024. Update in: PLoS One. 2025 Jul 3;20(7):e0327622. doi: 10.1371/journal.pone.0327622. PMID: 39484372 Free PMC article. Updated. Preprint.
-
Phosgene oxime: Injury and associated mechanisms compared to vesicating agents sulfur mustard and lewisite.Toxicol Lett. 2018 Sep 1;293:112-119. doi: 10.1016/j.toxlet.2017.11.011. Epub 2017 Nov 12. Toxicol Lett. 2018. PMID: 29141200 Free PMC article. Review.
-
Wnt activation as a potential therapeutic approach to treat partial limbal stem cell deficiency.Sci Rep. 2023 Sep 21;13(1):15670. doi: 10.1038/s41598-023-42794-8. Sci Rep. 2023. PMID: 37735479 Free PMC article.
References
-
- Geraci MJ. Mustard gas: imminent danger or eminent threat? Ann Pharmacother. 2008;42:237–246. - PubMed
-
- Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clin Exp Dermatol. 2006;31:1–5. - PubMed
-
- Smith KJ, Skelton H. Chemical warfare agents: their past and continuing threat and evolving therapies. Part I of II. Skinmed. 2003;2:215–221. - PubMed
-
- Ruff AL, Jarecke AJ, Hilber DJ, et al. Development of a mouse model for sulfur mustard-induced ocular injury and long-term clinical analysis of injury progression. Cutan Ocul Toxicol. 2013;32:140–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

