Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl-Lewis(x) to increase binding to airway epithelial cells
- PMID: 26555707
- PMCID: PMC4864173
- DOI: 10.1038/mi.2015.119
Pseudomonas aeruginosa pyocyanin modulates mucin glycosylation with sialyl-Lewis(x) to increase binding to airway epithelial cells
Abstract
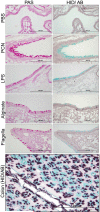
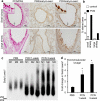
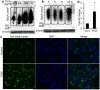
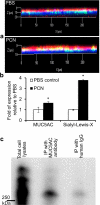
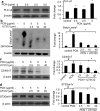
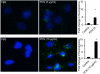
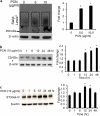
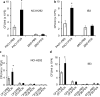
Cystic fibrosis (CF) patients battle life-long pulmonary infections with the respiratory pathogen Pseudomonas aeruginosa (PA). An overabundance of mucus in CF airways provides a favorable niche for PA growth. When compared with that of non-CF individuals, mucus of CF airways is enriched in sialyl-Lewis(x), a preferred binding receptor for PA. Notably, the levels of sialyl-Lewis(x) directly correlate with infection severity in CF patients. However, the mechanism by which PA causes increased sialylation remains uncharacterized. In this study, we examined the ability of PA virulence factors to modulate sialyl-Lewis(x) modification in airway mucins. We found pyocyanin (PCN) to be a potent inducer of sialyl-Lewis(x) in both mouse airways and in primary and immortalized CF and non-CF human airway epithelial cells. PCN increased the expression of C2/4GnT and ST3Gal-IV, two of the glycosyltransferases responsible for the stepwise biosynthesis of sialyl-Lewis(x), through a tumor necrosis factor (TNF)-α-mediated phosphoinositol-specific phospholipase C (PI-PLC)-dependent pathway. Furthermore, PA bound more efficiently to airway epithelial cells pre-exposed to PCN in a flagellar cap-dependent manner. Importantly, antibodies against sialyl-Lewis(x) and anti-TNF-α attenuated PA binding. These results indicate that PA secretes PCN to induce a favorable environment for chronic colonization of CF lungs by increasing the glycosylation of airway mucins with sialyl-Lewis(x).
Figures

References
-
- Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv. Drug Deliver. Rev. 2002;54:1359–1371. - PubMed
-
- Burns JL, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 2001;183:444–452. - PubMed
-
- Lamblin G, et al. Human airway mucin glycosylation: a combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconjugate J. 2001;18:661–684. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

