Intra-Section Analysis of Human Coronary Arteries Reveals a Potential Role for Micro-Calcifications in Macrophage Recruitment in the Early Stage of Atherosclerosis
- PMID: 26555788
- PMCID: PMC4640818
- DOI: 10.1371/journal.pone.0142335
Intra-Section Analysis of Human Coronary Arteries Reveals a Potential Role for Micro-Calcifications in Macrophage Recruitment in the Early Stage of Atherosclerosis
Abstract
Background: Vascular calcification is associated with poor cardiovascular outcome. Histochemical analysis of calcification and the expression of proteins involved in mineralization are usually based on whole section analysis, thereby often ignoring regional differences in atherosclerotic lesions. At present, limited information is available about factors involved in the initiation and progression of atherosclerosis.
Aim of this study: This study investigates the intra-section association of micro-calcifications with markers for atherosclerosis in randomly chosen section areas of human coronary arteries. Moreover, the possible causal relationship between calcifying vascular smooth muscle cells and inflammation was explored in vitro.
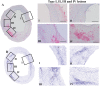
Technical approach: To gain insights into the pathogenesis of atherosclerosis, we performed analysis of the distribution of micro-calcifications using a 3-MeV proton microbeam. Additionally, we performed systematic analyses of 30 to 40 regions of 12 coronary sections obtained from 6 patients including histology and immuno-histochemistry. Section areas were classified according to CD68 positivity. In vitro experiments using human vascular smooth muscle cells (hVSMCs) were performed to evaluate causal relationships between calcification and inflammation.
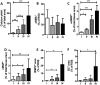
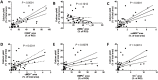
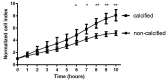
Results: From each section multiple areas were randomly chosen and subsequently analyzed. Depositions of calcium crystals at the micrometer scale were already observed in areas with early pre-atheroma type I lesions. Micro-calcifications were initiated at the elastica interna concomitantly with upregulation of the uncarboxylated form of matrix Gla-protein (ucMGP). Both the amount of calcium crystals and ucMGP staining increased from type I to IV atherosclerotic lesions. Osteochondrogenic markers BMP-2 and osteocalcin were only significantly increased in type IV atheroma lesions, and at this stage correlated with the degree of calcification. From atheroma area type III onwards a considerable number of CD68 positive cells were observed in combination with calcification, suggesting a pro-inflammatory effect of micro-calcifications. In vitro, invasion assays revealed chemoattractant properties of cell-culture medium of calcifying vascular smooth muscle cells towards THP-1 cells, which implies pro-inflammatory effect of calcium deposits. Additionally, calcifying hVSMCs revealed a pro-inflammatory profile as compared to non-calcifying hVSMCs.
Conclusion: Our data indicate that calcification of VSMCs is one of the earliest events in the genesis of atherosclerosis, which strongly correlates with ucMGP staining. Our findings suggest that loss of calcification inhibitors and/or failure of inhibitory capacity is causative for the early precipitation of calcium, with concomitant increased inflammation followed by osteochondrogenic transdifferentiation of VSMCs.
Conflict of interest statement
Figures


Similar articles
-
Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability.Atherosclerosis. 2020 Aug;306:85-95. doi: 10.1016/j.atherosclerosis.2020.05.017. Epub 2020 Jun 14. Atherosclerosis. 2020. PMID: 32654790 Review.
-
Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system.Arterioscler Thromb Vasc Biol. 2015 Feb;35(2):399-408. doi: 10.1161/ATVBAHA.114.304823. Epub 2014 Dec 23. Arterioscler Thromb Vasc Biol. 2015. PMID: 25538207
-
Natural progression of atherosclerosis from pathologic intimal thickening to late fibroatheroma in human coronary arteries: A pathology study.Atherosclerosis. 2015 Aug;241(2):772-82. doi: 10.1016/j.atherosclerosis.2015.05.011. Epub 2015 May 19. Atherosclerosis. 2015. PMID: 26058741 Free PMC article.
-
Calcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophages.J Pathol. 1998 May;185(1):10-7. doi: 10.1002/(SICI)1096-9896(199805)185:1<10::AID-PATH71>3.0.CO;2-0. J Pathol. 1998. PMID: 9713354
-
Vascular smooth muscle cells and calcification in atherosclerosis.Am Heart J. 2004 May;147(5):808-14. doi: 10.1016/j.ahj.2003.10.047. Am Heart J. 2004. PMID: 15131535 Review.
Cited by
-
Visceral and Ectopic Abdominal Fat Effect on the Calcification of the Abdominal Aorta and Its Branches-An MSCT Study.Life (Basel). 2023 Dec 19;14(1):2. doi: 10.3390/life14010002. Life (Basel). 2023. PMID: 38276251 Free PMC article.
-
The Elusive Origin of Atherosclerotic Plaque Calcification.Front Cell Dev Biol. 2021 Mar 9;9:622736. doi: 10.3389/fcell.2021.622736. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33768090 Free PMC article. Review.
-
Target identification for the diagnosis and intervention of vulnerable atherosclerotic plaques beyond 18F-fluorodeoxyglucose positron emission tomography imaging: promising tracers on the horizon.Eur J Nucl Med Mol Imaging. 2019 Jan;46(1):251-265. doi: 10.1007/s00259-018-4176-z. Epub 2018 Oct 9. Eur J Nucl Med Mol Imaging. 2019. PMID: 30302506 Free PMC article.
-
The Pleiotropic Role of Vitamin K in Multimorbidity of Chronic Obstructive Pulmonary Disease.J Clin Med. 2023 Feb 5;12(4):1261. doi: 10.3390/jcm12041261. J Clin Med. 2023. PMID: 36835797 Free PMC article. Review.
-
Vascular Calcification in Chronic Kidney Disease: The Role of Vitamin K- Dependent Matrix Gla Protein.Front Med (Lausanne). 2020 Apr 24;7:154. doi: 10.3389/fmed.2020.00154. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32391368 Free PMC article. Review.
References
-
- Rosenhek R., Binder T., Porenta G., Lang I., Christ G., Schemper M., et al., Predictors of outcome in severe, asymptomatic aortic stenosis, N. Engl. J. Med. 343 (2000) 611–617. - PubMed
-
- Raggi P., Shaw L., Berman D., Callister T., Prognostic value of coronary artery calcium screening in subjects with and without diabetes, J Am Coll Cardiol. 43 (2004) 1663–1669. - PubMed
-
- Raggi P., Callister T., Shaw L., Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy, Arterioscler Thromb Vasc Biol. 24 (2004) 1272–1277. - PubMed
-
- Joshi N.V., Vesey A.T., Williams M.C., Shah A.S.V., Calvert P.A., Craighead F.H.M., et al., 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial, Lancet. 383 (2014) 705–713. 10.1016/S0140-6736(13)61754-7 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

