The Efficient Derivation of Trophoblast Cells from Porcine In Vitro Fertilized and Parthenogenetic Blastocysts and Culture with ROCK Inhibitor Y-27632
- PMID: 26555939
- PMCID: PMC4640852
- DOI: 10.1371/journal.pone.0142442
The Efficient Derivation of Trophoblast Cells from Porcine In Vitro Fertilized and Parthenogenetic Blastocysts and Culture with ROCK Inhibitor Y-27632
Abstract
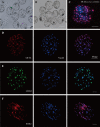
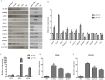
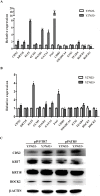
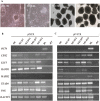
Trophoblasts (TR) are specialized cells of the placenta and play an important role in embryo implantation. The in vitro culture of trophoblasts provided an important tool to investigate the mechanisms of implantation. In the present study, porcine trophoblast cells were derived from pig in vitro fertilized (IVF) and parthenogenetically activated (PA) blastocysts via culturing in medium supplemented with KnockOut serum replacement (KOSR) and basic fibroblast growth factor (bFGF) on STO feeder layers, and the effect of ROCK (Rho-associated coiled-coil protein kinases) inhibiter Y-27632 on the cell lines culture was tested. 5 PA blastocyst derived cell lines and 2 IVF blastocyst derived cell lines have been cultured more than 20 passages; one PA cell lines reached 110 passages without obvious morphological alteration. The derived trophoblast cells exhibited epithelium-like morphology, rich in lipid droplets, and had obvious defined boundaries with the feeder cells. The cells were histochemically stained positive for alkaline phosphatase. The expression of TR lineage markers, such as CDX2, KRT7, KRT18, TEAD4, ELF5 and HAND1, imprinted genes such as IGF2, PEG1 and PEG10, and telomerase activity related genes TERC and TERF2 were detected by immunofluorescence staining, reverse transcription PCR and quantitative real-time PCR analyses. Both PA and IVF blastocysts derived trophoblast cells possessed the ability to differentiate into mature trophoblast cells in vitro. The addition of Y-27632 improved the growth of both PA and IVF blastocyst derived cell lines and increased the expression of trophoblast genes. This study has provided an alternative highly efficient method to establish trophoblast for research focused on peri-implantation and placenta development in IVF and PA embryos.
Conflict of interest statement
Figures



Similar articles
-
Establishment of bovine trophoblast stem-like cells from in vitro-produced blastocyst-stage embryos using two inhibitors.Stem Cells Dev. 2014 Jul 1;23(13):1501-14. doi: 10.1089/scd.2013.0329. Epub 2014 Apr 10. Stem Cells Dev. 2014. PMID: 24605918
-
Y-27632 enhances differentiation of blastocyst like cystic human embryoid bodies to endocrinologically active trophoblast cells on a biomimetic platform.J Biomed Sci. 2009 Sep 22;16(1):88. doi: 10.1186/1423-0127-16-88. J Biomed Sci. 2009. PMID: 19772618 Free PMC article.
-
Improvement in the blastocyst quality and efficiency of putative embryonic stem cell line derivation from porcine embryos produced in vitro using a novel culturing system.Mol Med Rep. 2015 Aug;12(2):2140-8. doi: 10.3892/mmr.2015.3634. Epub 2015 Apr 16. Mol Med Rep. 2015. PMID: 25892608
-
Human trophoblast stem cells: Real or not real?Placenta. 2017 Dec;60 Suppl 1(Suppl 1):S57-S60. doi: 10.1016/j.placenta.2017.01.003. Epub 2017 Jan 5. Placenta. 2017. PMID: 28087122 Free PMC article. Review.
-
Blastocysts don't go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells.Dev Biol. 2005 Apr 15;280(2):260-80. doi: 10.1016/j.ydbio.2005.02.009. Dev Biol. 2005. PMID: 15882572 Free PMC article. Review.
Cited by
-
Hemochorial placentation: development, function, and adaptations.Biol Reprod. 2018 Jul 1;99(1):196-211. doi: 10.1093/biolre/ioy049. Biol Reprod. 2018. PMID: 29481584 Free PMC article. Review.
-
ROCK Inhibitor (Y-27632) Abolishes the Negative Impacts of miR-155 in the Endometrium-Derived Extracellular Vesicles and Supports Embryo Attachment.Cells. 2022 Oct 10;11(19):3178. doi: 10.3390/cells11193178. Cells. 2022. PMID: 36231141 Free PMC article.
-
Defining cellular diversity at the swine maternal-fetal interface using spatial transcriptomics and organoids.PLoS Biol. 2025 Aug 28;23(8):e3003302. doi: 10.1371/journal.pbio.3003302. eCollection 2025 Aug. PLoS Biol. 2025. PMID: 40875599 Free PMC article.
-
Epigenetic regulation of BAF60A determines efficiency of miniature swine iPSC generation.Sci Rep. 2022 May 31;12(1):9039. doi: 10.1038/s41598-022-12919-6. Sci Rep. 2022. PMID: 35641537 Free PMC article.
-
miR-1246 is implicated as a possible candidate for endometrium remodelling facilitating implantation in buffalo (Bubalus bubalis).Vet Med Sci. 2023 Jan;9(1):443-456. doi: 10.1002/vms3.968. Epub 2022 Oct 25. Vet Med Sci. 2023. PMID: 36282011 Free PMC article.
References
-
- Niemann H, Rath D. Progress in reproductive biotechnology in swine. Theriogenology. 2001;56(8):1291–304. . - PubMed
-
- Kaufman MH. Early Mammalian Development: Parthenogenetic Studies In: Methodology: in vitro and vivo activation techniques. Cambridge: Cambridge University Press; 1983. 289 p.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

