Dissolution Rate Enhancement, Design and Development of Buccal Drug Delivery of Darifenacin Hydroxypropyl β-Cyclodextrin Inclusion Complexes
- PMID: 26556003
- PMCID: PMC4595963
- DOI: 10.1155/2013/983702
Dissolution Rate Enhancement, Design and Development of Buccal Drug Delivery of Darifenacin Hydroxypropyl β-Cyclodextrin Inclusion Complexes
Abstract
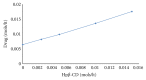
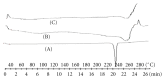
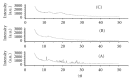
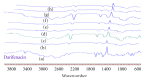
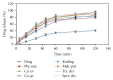
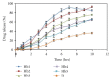
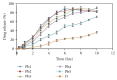
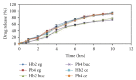
Darifenacin is a urinary antispasmodic. The oral absorption of darifenacin is poor due to its low solubility and poor bioavailability (15-19%). Darifenacin was complexed with hydroxylropyl beta-cyclodextrin (Hpβ-CD). The best results were obtained with the coevaporation that interacts in a 1 : 1 drug : cyclodextrin molar ratio. The solid inclusion complexes were found to be amorphous in the characterization. The dissolution rate of darifenacin from the Hpβ-CD solid inclusion complex was increased compared to the powdered drug. The controlled release buccoadhesive patches for the delivery of darifenacin were prepared using HPMC K100M CR and HPMC K15. The coevaporation complex of the drug was used in the formulation due to its increased saturation solubility and increased ease of dissolution. The patches were evaluated for their surface pH, folding endurance, swelling, mucoadhesive properties, in vitro residence time, vapour transmission test, and in vitro and ex vivo release studies. Formulations Hb2 (2%) and Pb4 (4%) were found to be optimized. These two formulations can be used for buccal delivery of darifenacin which avoids first pass effect and leads to increased bioavailability of darifenacin.
Figures
Similar articles
-
Formulation and evaluation of buccal film of Ivabradine hydrochloride for the treatment of stable angina pectoris.Int J Pharm Investig. 2013 Jan;3(1):47-53. doi: 10.4103/2230-973X.108963. Int J Pharm Investig. 2013. PMID: 23799205 Free PMC article.
-
Development of morin/hydroxypropyl-β-cyclodextrin inclusion complex: Enhancement of bioavailability, antihyperalgesic and anti-inflammatory effects.Food Chem Toxicol. 2019 Apr;126:15-24. doi: 10.1016/j.fct.2019.01.038. Epub 2019 Feb 6. Food Chem Toxicol. 2019. PMID: 30738132
-
Solubility and dissolution rate of progesterone-cyclodextrin-polymer systems.Drug Dev Ind Pharm. 2006 Oct;32(9):1043-58. doi: 10.1080/03639040600897093. Drug Dev Ind Pharm. 2006. PMID: 17012117
-
Influence of hydroxypropyl-beta-cyclodextrin complexation on piroxicam release from buccoadhesive tablets.Eur J Pharm Sci. 2004 Feb;21(2-3):251-60. doi: 10.1016/j.ejps.2003.10.029. Eur J Pharm Sci. 2004. PMID: 14757497
-
Development of a cyclodextrin-based nasal delivery system for lorazepam.Drug Dev Ind Pharm. 2008 Aug;34(8):817-26. doi: 10.1080/03639040801926063. Drug Dev Ind Pharm. 2008. PMID: 18622800
Cited by
-
Transbuccal delivery of betahistine dihydrochloride from mucoadhesive tablets with a unidirectional drug flow: in vitro, ex vivo and in vivo evaluation.Drug Des Devel Ther. 2016 Dec 14;10:4031-4045. doi: 10.2147/DDDT.S120613. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 28008227 Free PMC article.
-
Design and Evaluation of Inorganic/Organic Hybrid Bio-composite for Site-Specific Oral Delivery of Darifenacin.AAPS PharmSciTech. 2024 Sep 5;25(7):204. doi: 10.1208/s12249-024-02916-5. AAPS PharmSciTech. 2024. PMID: 39237789
References
-
- Verma S., Kaul M., Rawat A., Saini S. An overview on buccal drug delivery system. International Journal of Research in Pharmaceutical Sciences. 2011;2(4):1303–1321.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
