Symbiotic Human Gut Bacteria with Variable Metabolic Priorities for Host Mucosal Glycans
- PMID: 26556271
- PMCID: PMC4659458
- DOI: 10.1128/mBio.01282-15
Symbiotic Human Gut Bacteria with Variable Metabolic Priorities for Host Mucosal Glycans
Abstract
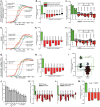
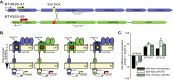
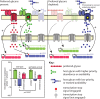
Many symbiotic gut bacteria possess the ability to degrade multiple polysaccharides, thereby providing nutritional advantages to their hosts. Like microorganisms adapted to other complex nutrient environments, gut symbionts give different metabolic priorities to substrates present in mixtures. We investigated the responses of Bacteroides thetaiotaomicron, a common human intestinal bacterium that metabolizes more than a dozen different polysaccharides, including the O-linked glycans that are abundant in secreted mucin. Experiments in which mucin glycans were presented simultaneously with other carbohydrates show that degradation of these host carbohydrates is consistently repressed in the presence of alternative substrates, even by B. thetaiotaomicron previously acclimated to growth in pure mucin glycans. Experiments with media containing systematically varied carbohydrate cues and genetic mutants reveal that transcriptional repression of genes involved in mucin glycan metabolism is imposed by simple sugars and, in one example that was tested, is mediated through a small intergenic region in a transcript-autonomous fashion. Repression of mucin glycan-responsive gene clusters in two other human gut bacteria, Bacteroides massiliensis and Bacteroides fragilis, exhibited variable and sometimes reciprocal responses compared to those of B. thetaiotaomicron, revealing that these symbionts vary in their preference for mucin glycans and that these differences occur at the level of controlling individual gene clusters. Our results reveal that sensing and metabolic triaging of glycans are complex processes that vary among species, underscoring the idea that these phenomena are likely to be hidden drivers of microbiota community dynamics and may dictate which microorganisms preferentially commit to various niches in a constantly changing nutritional environment.
Importance: Human intestinal microorganisms impact many aspects of health and disease, including digestion and the propensity to develop disorders such as inflammation and colon cancer. Complex carbohydrates are a major component of the intestinal habitat, and numerous species have evolved and refined strategies to compete for these coveted nutrients. Our findings reveal that individual bacteria exhibit different preferences for carbohydrates emanating from host diet and mucosal secretions and that some of these prioritization strategies are opposite to one another. Thus, we reveal new aspects of how individual bacteria, some with otherwise similar metabolic potential, partition to "preferred niches" in the complex gut ecosystem, which has important and immediate implications for understanding and predicting the behavioral dynamics of this community.
Copyright © 2015 Pudlo et al.
Figures


References
-
- McNeil NI. 1984. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr 39:338–342. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
