Insulin-Like Growth Factor (IGF)-I Modulates Endothelial Blood-Brain Barrier Function in Ischemic Middle-Aged Female Rats
- PMID: 26556536
- PMCID: PMC4701884
- DOI: 10.1210/en.2015-1840
Insulin-Like Growth Factor (IGF)-I Modulates Endothelial Blood-Brain Barrier Function in Ischemic Middle-Aged Female Rats
Abstract
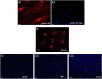
In comparison with young females, middle-aged female rats sustain greater cerebral infarction and worse functional recovery after stroke. These poorer stroke outcomes in middle-aged females are associated with an age-related reduction in IGF-I levels. Poststroke IGF-I treatment decreases infarct volume in older females and lowers the expression of cytokines in the ischemic hemisphere. IGF-I also reduces transfer of Evans blue dye to the brain, suggesting that this peptide may also promote blood-brain barrier function. To test the hypothesis that IGF-I may act at the blood-brain barrier in ischemic stroke, 2 approaches were used. In the first approach, middle-aged female rats were subjected to middle cerebral artery occlusion and treated with IGF-I after reperfusion. Mononuclear cells from the ischemic hemisphere were stained for CD4 or triple-labeled for CD4/CD25/FoxP3 and subjected to flow analyses. Both cohorts of cells were significantly reduced in IGF-I-treated animals compared with those in vehicle controls. Reduced trafficking of immune cells to the ischemic site suggests that blood-brain barrier integrity is better maintained in IGF-I-treated animals. The second approach directly tested the effect of IGF-I on barrier function of aging endothelial cells. Accordingly, brain microvascular endothelial cells from middle-aged female rats were cultured ex vivo and subjected to ischemic conditions (oxygen-glucose deprivation). IGF-I treatment significantly reduced the transfer of fluorescently labeled BSA across the endothelial monolayer as well as cellular internalization of fluorescein isothiocyanate-BSA compared with those in vehicle-treated cultures, Collectively, these data support the hypothesis that IGF-I improves blood-brain barrier function in middle-aged females.
Figures



References
-
- Andersen KK, Andersen ZJ, Olsen TS. Age- and gender-specific prevalence of cardiovascular risk factors in 40,102 patients with first-ever ischemic stroke: a Nationwide Danish Study. Stroke. 2010;41:2768–2774. - PubMed
-
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. - PubMed
-
- Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci. 2012;49:9–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

