Rapid chemical decontamination of infectious CJD and scrapie particles parallels treatments known to disrupt microbes and biofilms
- PMID: 26556670
- PMCID: PMC4826107
- DOI: 10.1080/21505594.2015.1098804
Rapid chemical decontamination of infectious CJD and scrapie particles parallels treatments known to disrupt microbes and biofilms
Abstract
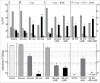
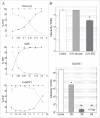
Neurodegenerative human CJD and sheep scrapie are diseases caused by several different transmissible encephalopathy (TSE) agents. These infectious agents provoke innate immune responses in the brain, including late-onset abnormal prion protein (PrP-res) amyloid. Agent particles that lack detectable PrP sequences by deep proteomic analysis are highly infectious. Yet these agents, and their unusual resistance to denaturation, are often evaluated by PrP amyloid disruption. To reexamine the intrinsic resistance of TSE agents to denaturation, a paradigm for less resistant viruses and microbes, we developed a rapid and reproducible high yield agent isolation procedure from cultured cells that minimized PrP amyloid and other cellular proteins. Monotypic neuronal GT1 cells infected with the FU-CJD or 22L scrapie agents do not have complex brain changes that can camouflage infectious particles and prevent their disruption, and there are only 2 reports on infectious titers of any human CJD strain treated with chemical denaturants. Infectious titers of both CJD and scrapie were reduced by >4 logs with Thiourea-urea, a treatment not previously tested. A mere 5 min exposure to 4M GdnHCl at 22°C reduced infectivity by >5 logs. Infectious 22L particles were significantly more sensitive to denaturation than FU-CJD particles. A protocol using sonication with these chemical treatments may effectively decontaminate complicated instruments, such as duodenoscopes that harbor additional virulent microbes and biofilms associated with recent iatrogenic infections.
Keywords: CJD; Guanidine HCl; Thiourea; Urea; decontamination; surgical instruments; viruses.
Figures




Similar articles
-
Replication and spread of CJD, kuru and scrapie agents in vivo and in cell culture.Virulence. 2011 May-Jun;2(3):188-99. doi: 10.4161/viru.2.3.15880. Epub 2011 May 1. Virulence. 2011. PMID: 21527829 Free PMC article.
-
Two Creutzfeldt-Jakob disease agents reproduce prion protein-independent identities in cell cultures.Proc Natl Acad Sci U S A. 2004 Jun 8;101(23):8768-73. doi: 10.1073/pnas.0400158101. Epub 2004 May 25. Proc Natl Acad Sci U S A. 2004. PMID: 15161970 Free PMC article.
-
Proteomic analysis of host brain components that bind to infectious particles in Creutzfeldt-Jakob disease.Proteomics. 2015 Sep;15(17):2983-98. doi: 10.1002/pmic.201500059. Epub 2015 Jun 9. Proteomics. 2015. PMID: 25930988 Free PMC article.
-
Prions--infectious pathogens causing the spongiform encephalopathies.CRC Crit Rev Clin Neurobiol. 1985;1(3):181-200. CRC Crit Rev Clin Neurobiol. 1985. PMID: 3915974 Review.
-
Molecular biology of prions causing infectious and genetic encephalopathies of humans as well as scrapie of sheep and BSE of cattle.Dev Biol Stand. 1991;75:55-74. Dev Biol Stand. 1991. PMID: 1686599 Review.
Cited by
-
Vaporized Hydrogen Peroxide and Ozone Gas Synergistically Reduce Prion Infectivity on Stainless Steel Wire.Int J Mol Sci. 2021 Mar 23;22(6):3268. doi: 10.3390/ijms22063268. Int J Mol Sci. 2021. PMID: 33806892 Free PMC article.
-
A prokaryotic viral sequence is expressed and conserved in mammalian brain.Proc Natl Acad Sci U S A. 2017 Jul 3;114(27):7118-7123. doi: 10.1073/pnas.1706110114. Epub 2017 Jun 19. Proc Natl Acad Sci U S A. 2017. PMID: 28630311 Free PMC article.
-
Native nanodiscs formed by styrene maleic acid copolymer derivatives help recover infectious prion multimers bound to brain-derived lipids.J Biol Chem. 2020 Jun 19;295(25):8460-8469. doi: 10.1074/jbc.RA119.012348. Epub 2020 May 1. J Biol Chem. 2020. PMID: 32358064 Free PMC article.
-
A Novel, Reliable and Highly Versatile Method to Evaluate Different Prion Decontamination Procedures.Front Bioeng Biotechnol. 2020 Oct 29;8:589182. doi: 10.3389/fbioe.2020.589182. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33195153 Free PMC article.
References
-
- Prusiner SB. Molecular biology of prion diseases. Science 1991; 252:1515-22; PMID:1675487; http://dx.doi.org/10.1126/science.1675487 - DOI - PubMed
-
- Prusiner S. Prions. Proc Natl Acad Sci USA 1998; 95:13363-83; PMID:9811807; http://dx.doi.org/10.1073/pnas.95.23.13363 - DOI - PMC - PubMed
-
- Manuelidis L, Chakrabarty T, Miyazawa K, Nduom NA, Emmerling K. The kuru infectious agent is a unique geographic isolate distinct from Creutzfeldt-Jakob disease and scrapie agents. Proc Natl Acad Sci U S A 2009; 106:13529-34; PMID:19633190; http://dx.doi.org/10.1073/pnas.0905825106 - DOI - PMC - PubMed
-
- Manuelidis L. Infectious particles, stress, and induced prion amyloids: a unifying perspective. Virulence 2013; 4:373-83; PMID:23633671; http://dx.doi.org/10.4161/viru.24838 - DOI - PMC - PubMed
-
- Kimberlin RH, Walker CA, Fraser H. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol 1989; 70:2017-25; PMID:2504883; http://dx.doi.org/10.1099/0022-1317-70-8-2017 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
