Effect of ceritinib (LDK378) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo
- PMID: 26556876
- PMCID: PMC4792582
- DOI: 10.18632/oncotarget.5989
Effect of ceritinib (LDK378) on enhancement of chemotherapeutic agents in ABCB1 and ABCG2 overexpressing cells in vitro and in vivo
Abstract
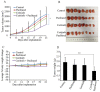
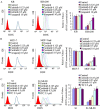
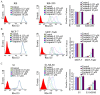
Multidrug resistance (MDR) is the leading cause of treatment failure in cancer chemotherapy. The overexpression of ATP-binding cassette (ABC) transporters, particularly ABCB1, ABCC1 and ABCG2, play a key role in mediating MDR by pumping anticancer drugs out from cancer cells. Ceritinib (LDK378) is a second-generation tyrosine kinase inhibitor of anaplastic lymphoma kinase (ALK) currently in phase III clinical trial for the treatment of non-small cell lung cancer. Here, we found that ceritinib remarkably enhanced the efficacy of chemotherapeutic drugs in ABCB1 or ABCG2 over-expressing cancer cells in vitro and in vivo. Ceritinib significantly increased the intracellular accumulation of chemotherapeutic agents such as doxorubicin (DOX) by inhibiting ABCB1 or ABCG2-mediated drug efflux in the transporters-overexpressing cells. Mechanistically, ceritinib is likely a competitive inhibitor of ABCB1 and ABCG2 because it competed with [(125)I]-iodoarylazidoprazosin for photo affinity labeling of the transporters. On the other hand, at the transporters-inhibiting concentrations, ceritinib did not alter the expression level of ABCB1 and ABCG2, and phosphorylation status of AKT and ERK1/2. Thus the findings advocate further clinical investigation of combination chemotherapy of ceritinib and other conventional chemotherapeutic drugs in chemo-refractory cancer patients.
Keywords: ABCB1; ABCG2; ATP-binding cassette transporters; ceritinib; multidrug resistance.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures




Similar articles
-
Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2.Cancer Lett. 2009 Jun 28;279(1):74-83. doi: 10.1016/j.canlet.2009.01.027. Epub 2009 Feb 18. Cancer Lett. 2009. PMID: 19232821
-
Ceritinib Enhances the Efficacy of Substrate Chemotherapeutic Agent in Human ABCB1-Overexpressing Leukemia Cells In Vitro, In Vivo and Ex-Vivo.Cell Physiol Biochem. 2018;46(6):2487-2499. doi: 10.1159/000489655. Epub 2018 May 5. Cell Physiol Biochem. 2018. PMID: 29742496
-
Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2.Cancer Res. 2008 Oct 1;68(19):7905-14. doi: 10.1158/0008-5472.CAN-08-0499. Cancer Res. 2008. PMID: 18829547 Free PMC article.
-
ABCG2: the key to chemoresistance in cancer stem cells?Expert Opin Drug Metab Toxicol. 2009 Dec;5(12):1529-42. doi: 10.1517/17425250903228834. Expert Opin Drug Metab Toxicol. 2009. PMID: 19708828 Review.
-
Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins.Drug Resist Updat. 2017 May;32:23-46. doi: 10.1016/j.drup.2017.10.003. Epub 2017 Oct 16. Drug Resist Updat. 2017. PMID: 29145976 Review.
Cited by
-
Adagrasib, a KRAS G12C inhibitor, reverses the multidrug resistance mediated by ABCB1 in vitro and in vivo.Cell Commun Signal. 2022 Sep 14;20(1):142. doi: 10.1186/s12964-022-00955-8. Cell Commun Signal. 2022. PMID: 36104708 Free PMC article.
-
New Therapeutic Strategy for Overcoming Multidrug Resistance in Cancer Cells with Pyrazolo[3,4-d]pyrimidine Tyrosine Kinase Inhibitors.Cancers (Basel). 2021 Oct 22;13(21):5308. doi: 10.3390/cancers13215308. Cancers (Basel). 2021. PMID: 34771471 Free PMC article.
-
Nobiletin Enhances Chemosensitivity to Adriamycin through Modulation of the Akt/GSK3β/β⁻Catenin/MYCN/MRP1 Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells.Nutrients. 2018 Nov 26;10(12):1829. doi: 10.3390/nu10121829. Nutrients. 2018. PMID: 30486290 Free PMC article.
-
Scaffold fragmentation and substructure hopping reveal potential, robustness, and limits of computer-aided pattern analysis (C@PA).Comput Struct Biotechnol J. 2021 May 10;19:3269-3283. doi: 10.1016/j.csbj.2021.05.018. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34141145 Free PMC article.
-
A curated binary pattern multitarget dataset of focused ATP-binding cassette transporter inhibitors.Sci Data. 2022 Jul 26;9(1):446. doi: 10.1038/s41597-022-01506-z. Sci Data. 2022. PMID: 35882865 Free PMC article.
References
-
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. - PubMed
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. - PubMed
-
- Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. - PubMed
-
- Miyake K, Mickley L, Litman T, Zhan Z, Robey R, Cristensen B, Brangi M, Greenberger L, Dean M, Fojo T, Bates SE. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

