The coordination dynamics of social neuromarkers
- PMID: 26557067
- PMCID: PMC4617382
- DOI: 10.3389/fnhum.2015.00563
The coordination dynamics of social neuromarkers
Abstract
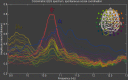
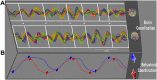
Social behavior is a complex integrative function that entails many aspects of the brain's sensory, cognitive, emotional and movement capacities. Its neural processes are seldom simultaneous but occur according to precise spatiotemporal choreographies, manifested by the coordination of their oscillations within and between brains. Methods with good temporal resolution can help to identify so-called "neuromarkers" of social function and aid in disentangling the dynamical architecture of social brains. In our ongoing research, we have used dual-electroencephalography (EEG) to study neuromarker dynamics during synchronic interactions in which pairs of subjects coordinate behavior spontaneously and intentionally (social coordination) and during diachronic transactions that require subjects to perceive or behave in turn (action observation, delayed imitation). In this paper, after outlining our dynamical approach to the neurophysiological basis of social behavior, we examine commonalities and differences in the neuromarkers that are recruited for both kinds of tasks. We find the neuromarker landscape to be task-specific: synchronic paradigms of social coordination reveal medial mu, alpha and the phi complex as contributing neuromarkers. Diachronic tasks recruit alpha as well, in addition to lateral mu rhythms and the newly discovered nu and kappa rhythms whose functional significance is still unclear. Social coordination, observation, and delayed imitation share commonality of context: in each of our experiments, subjects exchanged information through visual perception and moved in similar ways. Nonetheless, there was little overlap between their neuromarkers, a result that hints strongly of task-specific neural mechanisms for social behavior. The only neuromarker that transcended both synchronic and diachronic social behaviors was the ubiquitous alpha rhythm, which appears to be a key signature of visually-mediated social behaviors. The present paper is both an entry point and a challenge: much work remains to determine the nature and scope of recruitment of other neuromarkers, and to create theoretical models of their within- and between-brain dynamics during social interaction.
Keywords: alpha; brain rhythms; complexity; coordination dynamics; mu; phi complex; social coordination.
Figures





Similar articles
-
Functional dissociation of brain rhythms in social coordination.Clin Neurophysiol. 2012 Sep;123(9):1789-97. doi: 10.1016/j.clinph.2012.02.065. Epub 2012 Mar 17. Clin Neurophysiol. 2012. PMID: 22425484
-
The phi complex as a neuromarker of human social coordination.Proc Natl Acad Sci U S A. 2007 May 8;104(19):8190-5. doi: 10.1073/pnas.0611453104. Epub 2007 Apr 30. Proc Natl Acad Sci U S A. 2007. PMID: 17470821 Free PMC article.
-
Spectral dissociation of lateralized brain rhythms.Neurosci Res. 2020 Jul;156:141-146. doi: 10.1016/j.neures.2019.12.006. Epub 2019 Dec 26. Neurosci Res. 2020. PMID: 31883870 Free PMC article.
-
Neuromarkers in addiction: definitions, development strategies, and recent advances.J Neural Transm (Vienna). 2024 May;131(5):509-523. doi: 10.1007/s00702-024-02766-2. Epub 2024 Apr 17. J Neural Transm (Vienna). 2024. PMID: 38630190 Review.
-
The functional significance of mu rhythms: translating "seeing" and "hearing" into "doing".Brain Res Brain Res Rev. 2005 Dec 1;50(1):57-68. doi: 10.1016/j.brainresrev.2005.04.005. Epub 2005 May 31. Brain Res Brain Res Rev. 2005. PMID: 15925412 Review.
Cited by
-
How Interpersonal Coordination Affects Individual Behavior (and Vice Versa): Experimental analysis and adaptive HKB model of social memory.Ecol Psychol. 2018;30(3):224-249. doi: 10.1080/10407413.2018.1438196. Epub 2018 Mar 20. Ecol Psychol. 2018. PMID: 33041602 Free PMC article.
-
Integration and Causality in Enactive Approaches to Psychiatry.Front Psychiatry. 2022 Jul 4;13:870122. doi: 10.3389/fpsyt.2022.870122. eCollection 2022. Front Psychiatry. 2022. PMID: 35859602 Free PMC article.
-
Commentary: Hand and Grasp Selection in a Preferential Reaching Task: The Effects of Object Location, Orientation, and Task Intention.Front Psychol. 2016 Jul 28;7:1129. doi: 10.3389/fpsyg.2016.01129. eCollection 2016. Front Psychol. 2016. PMID: 27515474 Free PMC article. No abstract available.
-
Pain control based on oscillatory brain activity using transcranial alternating current stimulation: An integrative review.Front Hum Neurosci. 2023 Jan 19;17:941979. doi: 10.3389/fnhum.2023.941979. eCollection 2023. Front Hum Neurosci. 2023. PMID: 36742359 Free PMC article. Review.
-
More than Meets the Mind's Eye? Preliminary Observations Hint at Heterogeneous Alpha Neuromarkers for Visual Attention.Brain Sci. 2019 Nov 2;9(11):307. doi: 10.3390/brainsci9110307. Brain Sci. 2019. PMID: 31684067 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

