Inflammatory stress potentiates emodin-induced liver injury in rats
- PMID: 26557087
- PMCID: PMC4615941
- DOI: 10.3389/fphar.2015.00233
Inflammatory stress potentiates emodin-induced liver injury in rats
Erratum in
-
Corrigendum: Inflammatory Stress Potentiates Emodin-Induced Liver Injury in Rats.Front Pharmacol. 2021 Jan 25;11:597772. doi: 10.3389/fphar.2020.597772. eCollection 2020. Front Pharmacol. 2021. PMID: 33568998 Free PMC article.
Abstract
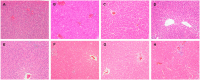
Herbal medicines containing emodin, widely used for the treatment of hepatitis in clinic, have been reported with hepatotoxicity in individuals. A modest inflammatory stress potentiating liver injury has been linked to the idiosyncratic drug-induced liver injury (IDILI). In this study, we investigated the hypothesis that lipopolysaccharide (LPS) interacts with emodin could synergize to cause liver injury in rats. Emodin (ranging from 20, 40, to 80 mg/kg), which is in the range of liver protection, was administered to rats, before LPS (2.8 mg/kg) or saline vehicle treatment. The biochemical tests showed that non-toxic dosage of LPS coupled with emodin caused significant increases of plasma ALT and AST activities as compared to emodin alone treated groups (P < 0.05). In addition, with LPS or emodin alone could not induce any changes in ALT and AST activity, as compared with the control group (0.5% CMC-Na treatment). Meanwhile, the plasma proinflammatory cytokines, TNF-α, IL-1β, and IL-6 increased significantly in the emodin/LPS groups compared to either emodin groups or the LPS (P < 0.05). Histological analysis showed that liver damage was only found in emodin/LPS cotreatmented rat livers samples. These results indicate that non-toxic dosage of LPS potentiates the hepatotoxicity of emodin. This discovery raises the possibility that emodin and herbal medicines containing it may induce liver injury in the inflammatory stress even in their therapeutic dosages.
Keywords: emodin; hepatotoxicity; idiosyncratic drug-induced liver injury; lipopolysaccharide; proinflammatory mediators; therapeutic dosages.
Figures
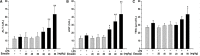
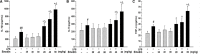
References
-
- Cao Q., Wang X. L. (2013). Study and applications of rhubarb licorice root decoctions. Chin. Med. Pharm. 3, 29–30.
LinkOut - more resources
Full Text Sources
Other Literature Sources

