Cell-based Hyper-interleukin 6 or Hyper-interleukin 11 secreting vaccines combined with low dose cyclophosphamide in an orthotopic murine prostate cancer model
- PMID: 26557758
- PMCID: PMC4631279
- DOI: 10.5114/wo.2015.52711
Cell-based Hyper-interleukin 6 or Hyper-interleukin 11 secreting vaccines combined with low dose cyclophosphamide in an orthotopic murine prostate cancer model
Abstract
Background: Cell based vaccines encoding Hyper-IL-6 (H6) and Hyper-IL-11 (H11) present high activity in murine melanoma and renal cancer model. We evaluated the efficacy of cellular vaccines modified with H6 or H11 combined with cyclophosphamide in orthotopic murine prostate cancer model.
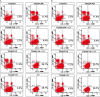
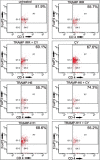
Material and methods: TRAMP cells were transduced with H6 and H11 cDNA (TRAMP-H6 and TRAMP-H11). An orthotopic TRAMP model based on the implantation of TRAMP cells into the dorsolateral lobe of the prostate of C57BL6/J mice was employed. The efficacy of TRAMP-H6 and TRAMP-H11 vaccines evaluated in the therapeutic setting was compared with the TRAMP cells modified with a mock transduced E1-deleted adenoviral vector (TRAMP-AdV) and non-modified irradiated TRAMP cells (TRAMP IRR) in relation to naive (non-immunized) mice. In the next experimental groups mice vaccinated with TRAMP-H6 and TRAMP-H11 received cyclophosphamide (CY). Detection of immune cells in the spleen in mice receiving vaccines combined with CY was evaluated.
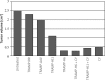
Results: Modification of TRAMP cells with H6 increased the efficacy of TRAMP-based whole-cell vaccine. The highest response rate was observed in mice receiving TRAMP-H6 alone and combined with CY. Vaccination with TRAMP-H6 alone and combined with CY and TRAMP H11 combined with CY extended median OS of mice bearing orthotopic TRAMP tumors in therapeutic setting. Low dose CY administered alone demonstrated some antitumor activity in employed model. TRAMP-H6 or TRAMP-H11 combined with CY strongly augmented generation of CD8+, CD4+ T lymphocytes and memory T cells. Immunization with TRAMP combined with or without CY suppressed generation of T regulatory cells.
Conslusions: Prostate cancer vaccines modified with H6 or H11 induce prostate tumour regression and increase mice survival by stimulating the immune system. Cyclophosphamide added to modified TRAMP vaccines demonstrated clinical benefit of treated mice and enhanced anti-tumour immune response.
Keywords: cancer vaccine; cyclophosphamide; immunotherapy; murine model; prostate cancer.
Figures

Similar articles
-
Cellular Vaccines Modified with Hyper IL6 or Hyper IL11 Combined with Docetaxel in an Orthotopic Prostate Cancer Model.Anticancer Res. 2015 Jun;35(6):3275-88. Anticancer Res. 2015. PMID: 26026087
-
Gene-modified tumor vaccine secreting a designer cytokine Hyper-Interleukin-6 is an effective therapy in mice bearing orthotopic renal cell cancer.Cancer Gene Ther. 2010 Jul;17(7):465-75. doi: 10.1038/cgt.2010.2. Epub 2010 Feb 19. Cancer Gene Ther. 2010. PMID: 20168352
-
Hyper-interleukin-11 novel designer molecular adjuvant targeting gp130 for whole cell cancer vaccines.Expert Opin Biol Ther. 2011 Dec;11(12):1555-67. doi: 10.1517/14712598.2011.627852. Epub 2011 Oct 14. Expert Opin Biol Ther. 2011. PMID: 21995459 Review.
-
[New strategy of cancer immunotherapy: irradiation or chemotherapeutics-induced lymphopenia combined with immune reconstitution and tumor vaccine].Zhonghua Zhong Liu Za Zhi. 2005 Aug;27(8):452-6. Zhonghua Zhong Liu Za Zhi. 2005. PMID: 16188138 Chinese.
-
Therapeutic gene modified cell based cancer vaccines.Gene. 2013 Aug 10;525(2):200-7. doi: 10.1016/j.gene.2013.03.056. Epub 2013 Apr 6. Gene. 2013. PMID: 23566846 Review.
Cited by
-
Re-induction using whole cell melanoma vaccine genetically modified to melanoma stem cells-like beyond recurrence extends long term survival of high risk resected patients - updated results.J Immunother Cancer. 2018 Nov 29;6(1):134. doi: 10.1186/s40425-018-0456-1. J Immunother Cancer. 2018. PMID: 30486884 Free PMC article. Clinical Trial.
References
-
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. - PubMed
-
- Fong L, Small EJ. Immunotherapy for prostate cancer. Curr Oncol Rep. 2007;9:226–33. - PubMed
-
- Mach N, Dranoff G. Cytokine-secreting tumor cell vacines. Curr Opin Immunol. 2000;12:571–5. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
