Optimal management of hormone receptor positive metastatic breast cancer in 2016
- PMID: 26557899
- PMCID: PMC4622303
- DOI: 10.1177/1758834015608993
Optimal management of hormone receptor positive metastatic breast cancer in 2016
Abstract
Hormone receptor positive tumors represent the most common form of breast cancer and account for most of the deaths from the disease. Endocrine therapy represents the main initial therapeutic strategy for these patients and has been associated with significant clinical benefits in a majority of patients. While in early stages endocrine therapy is administered as part of a curative approach once clinical metastases develop, the disease is considered incurable and the main management objectives are tumor control and quality of life. The two major clinical paradigms of always indicating endocrine therapy in the absence of visceral crises and sequencing endocrine treatments have been guiding our therapeutic approach to these patients. However, for many decades, we have delivered endocrine therapy with a 'one size fits all' approach by applying agents that interfere with hormone receptor signaling equally in every clinical patient scenario. We have been unable to incorporate the well-known biologic principle of different degrees of hormone receptor dependency in our therapeutic recommendations. Recent developments in the understanding of molecular interactions of hormone signaling with other important growth factor, metabolic and cell division pathways have opened the possibility of improving results by modulating hormone signaling and interfering with resistance mechanisms yet to be fully understood. Unfortunately, limitations in the design of trials conducted in this area have made it difficult to develop predictive biomarkers and most of the new combinations with targeted agents, even though showing improvements in clinical endpoints, have been directed to an unselected population of patients. In this review we explore some of the current and most relevant literature in the management of hormone receptor positive advance breast cancer.
Keywords: advanced breast cancer; breast neoplasms; drug resistance; hormone receptor.
Conflict of interest statement
Figures
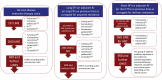
The sequence of the treatment alternatives in each line text box does not represent a particular preference order.
Subsequent use of ET should always take into account previous lines of treatment as well as the type and duration of response to previous ET.
Intrinsic or primary resistance has been defined as recurrence within the first 2 years of adjuvant ET or progressive disease within 6 months of starting ET in the advanced setting.
Acquired or secondary resistance has been defined as recurrence after the first 2 years of adjuvant ET or disease progression more than 6 months after initiation of ET in the advanced setting. These definitions, although imperfect and somewhat arbitrary, have been useful in some clinical trials to analyze and stratify patient populations.
These suggestions refer to postmenopausal patients. Specific management of the premenopausal population is addressed in the text.
These suggestions do not take into consideration acess and regulatory issues in the dfferent regions of the world.
References
-
- Abrams J., Aisner J., Cirrincione C., Berry D., Muss H., Cooper M., et al. (1999) Dose-response trial of megestrol acetate in advanced breast cancer: cancer and leukemia group B phase III study 8741. J Clin Oncol 17: 64–73. - PubMed
-
- Adelson K., Raptis G., Sparano J., Germain D. (2014) Randomized phase II study of fulvestrant versus fulvestrant plus bortezomib in postmenopausal women with estrogen receptor (ER) positive, aromatase-inhibitor (AI) resistant metastatic breast cancer (MBC): New York Cancer Consortium trial P8457. Cancer Res 71: OT3-01-01.
-
- Bachelot T., Bourgier C., Cropet C., Ray-Coquard I., Ferrero J., Freyer G., et al. (2012) Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol 30: 2718–2724. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

