Reversible Ceftriaxone-Induced Pseudolithiasis in an Adult Patient with Maintenance Hemodialysis
- PMID: 26558252
- PMCID: PMC4608644
- DOI: 10.1159/000440680
Reversible Ceftriaxone-Induced Pseudolithiasis in an Adult Patient with Maintenance Hemodialysis
Abstract
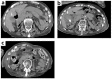
Ceftriaxone (CTRX) is a third-generation cephalosporin widely used for the treatment of bacterial infections in patients with renal disease because of its excretion by both renal and hepatic mechanisms. Biliary pseudolithiasis is a known CTRX-associated complication; however, there have been no studies of this adverse event in adult patients receiving maintenance hemodialysis. Here we report the case of a 79-year-old Japanese woman with end-stage renal disease (ESRD) receiving maintenance hemodialysis who developed CTRX-induced pseudolithiasis. The patient received CTRX for bronchial pneumonia. Fifteen days following CTRX initiation, the patient presented with stomachache. Because of the presence of one gallstone and increased gallbladder wall thickness on computed tomography scans, not detected at the onset of pneumonia, the patient was diagnosed with CTRX-induced gallbladder pseudolithiasis. CTRX was discontinued immediately. At 48 days following CTRX withdrawal, the gallstone and thickening of the gallbladder wall had completely resolved. ESRD may be a risk factor for CTRX-induced pseudolithiasis as hepatic excretion of CTRX is the predominant clearance mechanism in patients with ESRD. More attention should be paid to CTRX-induced pseudolithiasis following the use of CTRX in ESRD patients.
Keywords: Adult; Ceftriaxone; Maintenance hemodialysis; Pseudolithiasis.
Figures
References
-
- Arvidsson A, Alvan G, Angelin B, Borga O, Nord CE. Ceftriaxone: renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother. 1982;10:207–215. - PubMed
-
- Richards DM, Heel RC, Brogden RN, Speight TM, Avery GS. Ceftriaxone. A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs. 1984;27:469–527. - PubMed
-
- Schaad UB, Wedgwood-Krucko J, Tschaeppeler H. Reversible ceftriaxone-associated biliary pseudolithiasis in children. Lancet. 1988;2:1411–1413. - PubMed
-
- Pigrau C, Pahissa A, Gropper S, Sureda D, Martinez Vazquez JM. Ceftriaxone-associated biliary pseudolithiasis in adults. Lancet. 1989;2:165. - PubMed
-
- Shiffman ML, Keith FB, Moore EW. Pathogenesis of ceftriaxone-associated biliary sludge. In vitro studies of calcium-ceftriaxone binding and solubility. Gastroenterology. 1990;99:1772–1778. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

