CMP-Neu5Ac Hydroxylase Null Mice as a Model for Studying Metabolic Disorders Caused by the Evolutionary Loss of Neu5Gc in Humans
- PMID: 26558285
- PMCID: PMC4629002
- DOI: 10.1155/2015/830315
CMP-Neu5Ac Hydroxylase Null Mice as a Model for Studying Metabolic Disorders Caused by the Evolutionary Loss of Neu5Gc in Humans
Abstract
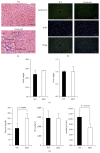
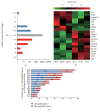
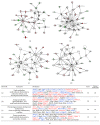
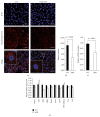
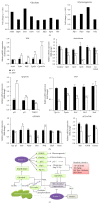
The purpose of this study was to identify the modification/turnover of gene products that are altered in humans due to evolutionary loss of Neu5Gc. CMP-Neu5Ac hydroxylase- (Cmah-) deficient mice show the infiltration of Kupffer cells within liver sinusoids, whereas body and liver weight develop normally. Pathway analysis by use of Illumina MouseRef-8 v2 Expression BeadChip provided evidence that a number of biological pathways, including the glycolysis, gluconeogenesis, TCA cycle, and pentose phosphate pathways, as well as glycogen metabolism-related gene expression, were significantly upregulated in Cmah-null mice. The intracellular glucose supply in Cmah-null mice resulted in mitochondrial dysfunction, oxidative stress, and the advanced glycation end products accumulation that could further induce oxidative stress. Finally, low sirtuin-1 and sirtuin-3 gene expressions due to higher NADH/NAD in Cmah-null mice decreased Foxo-1 and MnSOD gene expression, suggesting that oxidative stress may result in mitochondrial dysfunction in Cmah-null mouse. The present study suggests that mice with CMAH deficiency can be taken as an important model for studying metabolic disorders in humans.
Figures

References
-
- Brinkman-Van der Linden E. C. M., Sjoberg E. R., Juneja L. R., Crocker P. R., Varki N., Varki A. Loss of N-glycolylneuraminic acid in human evolution. Implications for sialic acid recognition by siglecs. The Journal of Biological Chemistry. 2000;275(12):8633–8640. doi: 10.1074/jbc.275.12.8633. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

