Antimicrobial Peptide Mimicking Primary Amine and Guanidine Containing Methacrylamide Copolymers Prepared by Raft Polymerization
- PMID: 26558609
- PMCID: PMC4825813
- DOI: 10.1021/acs.biomac.5b01162
Antimicrobial Peptide Mimicking Primary Amine and Guanidine Containing Methacrylamide Copolymers Prepared by Raft Polymerization
Abstract
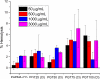
Naturally occurring antimicrobial peptides (AMPs) display the ability to eliminate a wide variety of bacteria, without toxicity to the host eukaryotic cells. Synthetic polymers containing moieties mimicking lysine and arginine components found in AMPs have been reported to show effectiveness against specific bacteria, with the mechanism of activity purported to depend on the nature of the amino acid mimic. In an attempt to incorporate the antimicrobial activity of both amino acids into a single water-soluble copolymer, a series of copolymers containing lysine mimicking aminopropyl methacrylamide (APMA) and arginine mimicking guanadinopropyl methacrylamide (GPMA) were prepared via aqueous RAFT polymerization. Copolymers were prepared with varying ratios of the comonomers, with degree of polymerization of 35-40 and narrow molecular weight distribution to simulate naturally occurring AMPs. Antimicrobial activity was determined against Gram-negative and Gram-positive bacteria under conditions with varying salt concentration. Toxicity to mammalian cells was assessed by hemolysis of red blood cells and MTT assays of MCF-7 cells. Antimicrobial activity was observed for APMA homopolymer and copolymers with low concentrations of GPMA against all bacteria tested, with low toxicity toward mammalian cells.
Figures



References
-
- Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003;55(1):27–55. - PubMed
-
- Yount NY, Yeaman MR. Emerging themes and therapeutic prospects for anti-infective peptides. Annu. Rev. Pharmacol. Toxicol. 2012;52:337–360. - PubMed
-
- Fjell CD, Hiss JA, Hancock REW, Schneider G. Designing antimicrobial peptides: form follows function. Nat. Rev. Neurosci. 2012;11(1):37–51. - PubMed
-
- Ilker MF, Nuesslein K, Tew GN, Coughlin EB. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 2004;126(48):15870–15875. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

